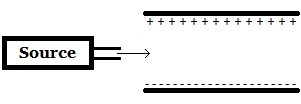|
| 1 |
Go |
Q:
|
The half-life of a radioactive element is 4 s. How long will it take for a sample of 64 g to decay to 2 g? |
|
A
|
24 s |
B
|
20 s |
C
|
32 s |
D
|
2 s |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 2 |
Go |
Q:
|
U-238 (Uranium of atomic number 92) undergoes alpha decay, followed by a series of 3 beta decays, followed by a final alpha decay and a gamma emission. How many neutrons will there theoretically be in this atom after the series of decays? |
|
|
|
|
Tags:
Nuclear Chemistry | |
|
| 3 |
Go |
Q:
|
Which of the following best describes a positron? |
|
A
|
positively charged electron |
B
|
positively charged neutron |
C
|
positively charged helium nucleus |
D
|
positively charged gamma emission |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 4 |
Go |
Q:
|
An alpha particle is the same as which of the following? |
|
A
|
positively charged electron |
B
|
negatively charged proton |
C
|
nucleus of a hydrogen atom |
D
|
nucleus of a helium atom |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 5 |
Go |
Q:
|
If an atom is shifted from an energy state of n=1 to n=3. If the energy state drops back from n=3 to n=1 at once, which of the following is most likely to be emitted? |
|
A
|
electron |
B
|
photon |
C
|
positron |
D
|
neutron |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 6 |
Go |
Q:
|
Through a lengthy decay process, compound X becomes compound Y. Compound X has 92 protons and an atomic mass of 238. Compound Y has 73 protons and an atomic mass of 198. Which of the following represents a potential decay reaction that could yield compound Y?
|
|
A
|
10 alpha decays |
B
|
5 alpha decays, followed by 1 beta decay, followed by 5 alpha decays
|
C
|
10 alpha decays followed by 2 gamma decays
|
D
|
5 alpha decays, followed by 1 electron capture, followed by 5 alpha decays
|
|
|
|
Tags:
Nuclear Chemistry | |
|
| 7 |
Go |
Q:
|
A new material is found which undergoes nuclear decay. It is found that the particles it emits are largely alpha particles. In a medical sense, why would it be dangerous for this material to be placed inside of a human body? |
|
A
|
The particles emitted have high ionizing power. |
B
|
The particles emitted have high penetration. |
C
|
Both A and B |
D
|
Neither A nor B |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 8 |
Go |
Q:
|
What is the major determinant of the half-life for a radioactive decay? |
|
A
|
The concentration of radioisotope |
B
|
The activation energy of the decay |
C
|
The temperature |
D
|
The difference in mass between the reactant and product |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 9 |
Go |
Q:
|
A particular organism well preserved in ice has been found to contain 5 mg of a radioactive particle X. Lifeforms usually contain around 40 mg of this particle. The half-life of X is around 20,000 years. Before this measurement of X, scientists believed that the organism was 100,000 years old. Their prediction was
|
|
A
|
Too low |
B
|
Correct |
C
|
Too high |
D
|
Cannot be confirmed or negated by the data |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 10 |
Go |
Q:
|
Substances X and Y are available to be used for resonance imaging in a patient. X releases beta particles and has a half-life of 3.4 years, while Y releases alpha particles and has a half-life of 2 ms. Which of the substances would be best for use in the imaging, considering that imaging works through the release of particles from the body, which are read by a machine outside the body.
|
|
A
|
Substance X |
B
|
Substance Y |
C
|
Both substances would be equally effective |
D
|
Neither substance would be effective |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 11 |
Go |
Q:
|
What kind of radiation will travel through an electric field on a pathway that remains unaffected by the field? |
|
A
|
a proton |
B
|
a gamma ray |
C
|
an electron |
D
|
an alpha particle |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 12 |
Go |
Q:
|
Radiation can affect cells in many different ways. Which of the following is the most likely consequence of a cell being irradiated with gamma radiation? |
|
A
|
DNA structure is altered |
B
|
Protein primary structure is altered |
C
|
Lipid is altered |
D
|
Water forms hydroxyl radical |
|
|
|
Tags:
DNA Replication and Repair | Nuclear Chemistry | |
|
| 13 |
Go |
Q:
|
What kind of radiation will travel through an electric field on a pathway that remains unaffected by the field? |
|
A
|
a proton |
B
|
a gamma ray |
C
|
an electron |
D
|
an alpha particle |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 14 |
Go |
Q:
|
Which of the following types of radiation is most likely to collide with molecules in epithelial cells? |
|
A
|
Alpha |
B
|
Beta |
C
|
Gamma |
D
|
Positron |
|
|
|
Tags:
Skin | Nuclear Chemistry | |
|
| 15 |
Go |
Q:
|
A particular radioisotope decays in the following order: alpha, beta, beta, alpha, gamma. How many neutrons have been emitted during the series of decays? |
|
|
|
|
Tags:
Nuclear Chemistry | |
|
| 16 |
Go |
Q:
|
112 g of an isotope are present at t = 0. At t = 18 minutes, 28 g of isotope remain. What is the half life of the isotope? |
|
A
|
4.5 minutes |
B
|
9 minutes |
C
|
18 minutes |
D
|
36 minutes |
|
|
|
Tags:
Nuclear Chemistry | Quantitative Skills | |
|
| 17 |
Go |
Q:
|
A particular organism well preserved in ice has been found to contain 1 mg of a radioactive isotope X. Lifeforms usually contain around 40 mg of this isotope. The half-life of X is around 20,000 years. For the organism with 1 mg of the isotope, scientists believe it died 100,000 years ago. Their prediction was |
|
A
|
~100,000 years too low |
B
|
~50,000 years too high |
C
|
Relatively accurate |
D
|
Unconfirmed |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 18 |
Go |
Q:
|
Workers routinely exposed to radiation are often required to wear special radiation-detecting badges when at work. These badges detect only certain types of ionizing radiation. The badges are constructed with radiation-sensitive material which is covered in a hard plastic covering and paper. Which type(s) of radiation are not likely detected by the badges?
I. UV radiation
II. Alpha emissions
III. Gamma radiation
|
|
A
|
I only |
B
|
I and II only |
C
|
II and III only |
D
|
I, II, and III |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 19 |
Go |
Q:
|
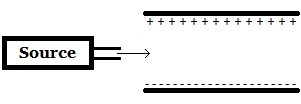
A beta particle (β-) is emitted from a source and travels between two plates as shown in the image above. Towards which plate (if any) will the beta particle move? |
|
A
|
toward the negatively charged plate |
B
|
toward the positively charged plate |
C
|
it will not move toward either plate |
D
|
it depends on the magnitude of the charges on the plates |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 20 |
Go |
Q:
|
Through a length decay process, compound X becomes compound Y. Compound X has 92 protons and an atomic mass of 238. Compound Y has 73 protons and an atomic mass of 198. Which of the following represents a potential decay reaction that could yield compound Y? |
|
A
|
10 alpha decays |
B
|
5 alpha decays, followed by 1 beta decay, followed by 5 alpha decays |
C
|
10 alpha decays followed by 2 gamma decays |
D
|
5 alpha decays, followed by 1 electron capture, followed by 5 alpha decays |
|
|
|
Tags:
Nuclear Chemistry | Quantitative Skills | |
|
| 21 |
Go |
Q:
|
An unknown substance was shown to have a half-life of 5.0 seconds. If you observe 12.5 g of the
substance after 15.0 seconds, how much of the substance was there initially? |
|
A
|
12.5 g |
B
|
25.0 g |
C
|
50.0 g |
D
|
100 g |
|
|
|
Tags:
Nuclear Chemistry | Quantitative Skills | |
|
| 22 |
Go |
Q:
|
Which of the following is an isotope of 13153I?
I. 12953I
II. 13155I
III. 12955I |
|
A
|
I only |
B
|
II only |
C
|
III only |
D
|
None of the listed atoms is an isotope of 13153I |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 23 |
Go |
Q:
|
In radioactive decay, electron capture serves which of the following purposes? |
|
A
|
Converts a proton to a neutron |
B
|
Ionizes the atom |
C
|
Converts a neutron to a proton |
D
|
Converts a neutron to an electron |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 24 |
Go |
Q:
|
Which of the following lab techniques use the concept of mass to charge ratio? |
|
A
|
Distillation |
B
|
Crystallization |
C
|
Mass Spectroscopy |
D
|
Nuclear Magnetic Resonance |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 25 |
Go |
Q:
|
In any given atom, what is the maximum number of electrons that can have the quantum number 3, 2, -2, 1/2? |
|
|
|
|
Tags:
Nuclear Chemistry | |
|
| 26 |
Go |
Q:
|
The advantage of coupling gas chromatography followed by mass spectrometry over simply mass spectrometry
is that |
|
A
|
the GC-MS is capable of separating individual analytes prior to fragmentation in the MS |
B
|
the GC-MS is capable of fragmenting all the analytes at once prior to analysis on the GC |
C
|
the GC-MS requires less time to operate than the GC alone |
D
|
neither technique provides useful information on its own; only together can their data be put to any use
|
|
|
|
Tags:
Nuclear Chemistry | |
|
| 27 |
Go |
Q:
|
A particular gas is placed in a cathode ray and energy is ran through the tube in the form of an electric current. A bright orange light can be seen once current is provided. Which of the following explains the phenomenon observed? |
|
A
|
Electrons of the gas become excited, which eventually results in the release of an electron with energy in the spectrum of visible light |
B
|
Gas molecules are excited and oscillate at a frequency in the visible light spectrum |
C
|
Photons are released after the excitation of electrons in the individual gas atoms |
D
|
Gas molecules absorb the energy and convert it into visible light through energy transfer within the nucleus |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 28 |
Go |
Q:
|
In the nuclear reaction
23892U → 23893Np + X
what type of nuclear decay has occurred? |
|
A
|
Electron capture |
B
|
Positron emission |
C
|
Gamma emission |
D
|
Beta decay |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 29 |
Go |
Q:
|
The isotope effect is commonly seen in mass spectra with small peaks directly adjacent to the main peaks for a particular ion. These isotope effects are seen due to differences in the number of
I. protons
II. neutrons
III. electrons |
|
A
|
II only |
B
|
I and II only |
C
|
I and III only |
D
|
I, II, and III |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 30 |
Go |
Q:
|
Which of the four fundamental forces holds the nucleus together? |
|
A
|
Weak Force |
B
|
Strong Force |
C
|
Electromagnetism |
D
|
Gravitation |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 31 |
Go |
Q:
|
24295Am undergoes β- radioactive decay, converting it into |
|
A
|
24295Am- |
B
|
24195Am |
C
|
24296Cm |
D
|
24294Pu |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 32 |
Go |
Q:
|
Suppose the half-life of 14C (currently 5730 years) has doubled in the last 50,000 years. When calculating the age of a specimen, if the changing rate is not taken into account when using 5730 as the half-life, how will the calculated age compare to the actual age? Note that the age is calculated after first determining the number of half-lives. |
|
A
|
The calculated age will be greater than the actual age because less 14C is present than there would be if the half-life had not changed |
B
|
The calculated age will be greater than the actual age because more 14C is present than there would be if the half-life had not changed |
C
|
The calculated age will be less than the actual age because less 14C is present than there would be if the half-life had not changed |
D
|
The calculated age will be less than the actual age because more 14C is present than there would be if the half-life had not changed |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 33 |
Go |
Q:
|
The nuclear reaction of 2613Al → 2612Mg is an example of which of the following types of nuclear decay? |
|
A
|
β- decay |
B
|
alpha decay |
C
|
gamma decay |
D
|
electron capture |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 34 |
Go |
Q:
|
In which of the following types of radioactive decay is there a change in the mass number of the isotope? |
|
A
|
electron capture |
B
|
β+ emission |
C
|
gamma emission |
D
|
alpha decay |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 35 |
Go |
Q:
|
Nitrogen is found as the isotopes Nitrogen-14 and Nitrogen-15 in nature. Which of the following statements is true? |
|
A
|
Both isotopes are found in equal amounts in nature |
B
|
Nitrogen-14 decays more rapidly than Nitrogen-15 |
C
|
Nitrogen-15 is unstable |
D
|
Nitrogen-14 makes up almost 100% of naturally occurring Nitrogen |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 36 |
Go |
Q:
|
Which of the following particles has the greatest momentum when traveling at 99% the speed of light? |
|
A
|
β- particle |
B
|
a neutron |
C
|
an α particle |
D
|
β+ particle |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 37 |
Go |
Q:
|
The half-life of 164Gd is 45 seconds. How long would it take a sample of 5 x 108 nuclei to decay to 2 x 106 nuclei? |
|
A
|
45 seconds |
B
|
3 minutes |
C
|
4.5 minutes |
D
|
6 minutes |
|
|
|
Tags:
Nuclear Chemistry | Quantitative Skills | |
|
| 38 |
Go |
Q:
|
Atomic nuclei can be bombarded together to form much heavier atoms for further study. In one experiment, researchers complete the following reaction:
24494Pu + 4820Ca → 292114X → 289114Y + 3 10n
Which of the following describes the relationship between X and Y? |
|
A
|
X and Y are different ions of the same element. |
B
|
X and Y are different elements. |
C
|
X and Y are different isotopes of the same element. |
D
|
X and Y are unrelated. |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 39 |
Go |
Q:
|
In a radioactive series, the nucleus of 24195Am decays and emits an alpha particle, β- decay, and then β- decay. The final product of the series is: |
|
A
|
24491Pa |
B
|
23793Np |
C
|
23795Am |
D
|
23791Pa |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 40 |
Go |
Q:
|
In radioactive decay alpha radiation can be shielded with a piece of paper, requiring far less shielding than gamma or beta radiation. This is primarily due to which of the following? |
|
A
|
Alpha particles are far more massive than other forms of radiation |
B
|
Alpha particles are not as harmful as other forms of radiation |
C
|
Alpha particles are uncharged and thus do not have high penetration abilities |
D
|
Alpha particles are emitted at a far higher energy than other forms of radiation |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 41 |
Go |
Q:
|
A radioactive isotope of 237Np emits two alpha particles and one β+ particle. What is the resultant isotope?
|
|
A
|
230Th |
B
|
229Ra |
C
|
230Pa |
D
|
233U |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 42 |
Go |
Q:
|
Which of the following would be most useful as a shield against gamma radiation? |
|
A
|
barium sulfate |
B
|
argon gas |
C
|
boron |
D
|
hydrochloric acid |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 43 |
Go |
Q:
|
Which of the following types of emissions are massless and have no charge? |
|
A
|
Neutron |
B
|
Gamma |
C
|
Alpha |
D
|
Beta |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 44 |
Go |
Q:
|
A particular atom has a mass number of 104 with 64 protons. How many alpha decays are necessary to get to a mass number of 84? |
|
|
|
|
Tags:
Nuclear Chemistry | |
|
| 45 |
Go |
Q:
|
For the nuclear reaction 4019K → 4020Ca, which of the following is missing from the products side? |
|
|
|
|
Tags:
Nuclear Chemistry | |
|
| 46 |
Go |
Q:
|
Acute neutron radiation poisoning can occur after exposure to a critical nuclear reactor accident. The most reliable way of measuring exposure levels to neutrons is to measure the blood levels of otherwise rare isotopes. Which of the following would be the best measure for neutron exposure? |
|
A
|
Presence of 116C versus normal 126C |
B
|
Presence of 189F versus normal 188O |
C
|
Presence of 2411Na versus normal 2311Na |
D
|
Presence of 12553I versus normal 12753I |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 47 |
Go |
Q:
|
If 209Po underwent alpha decay followed by β- decay, what would be the product? |
|
A
|
204Pb |
B
|
205Bi |
C
|
209At |
D
|
210Fr |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 48 |
Go |
Q:
|
When a 85B atom decays by the emission of a positron, the resultant atom is: |
|
A
|
84Be |
B
|
86C |
C
|
58B |
D
|
43Li |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 49 |
Go |
Q:
|
The only stable isotope of Scandium is 45Sc, but radioisotopes have been isolated that range from 36Sc to 60Sc. Isotopes heavier than 45Sc almost all use the same mode of decay to balance their proton:neutron ratio closer to 1:1. Which of the following decays do they undergo? |
|
A
|
alpha decay |
B
|
β- decay |
C
|
β+ decay |
D
|
gamma decay |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 50 |
Go |
Q:
|
Nuclear imaging is a radiology modality which utilizes radioisotopes to produce images of internal organs. Patients are injected with a a radioisotope; this isotope is taken up by various organs with differential specificity. Radioactive decay occurs and the particles transmitted are recorded by a receiver. For a successful study, it is necessary for the particle to travel a long distance out of the body and to the receiver. Which of the following types of emission is likely recorded? |
|
A
|
alpha decay |
B
|
beta decay |
C
|
positron emission |
D
|
gamma emission |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 51 |
Go |
Q:
|
Which of the following denotes the difference between an alpha particle and a helium atom? |
|
A
|
An alpha particle is the same as a helium atom |
B
|
Alpha particles carry no net charge, which differs from helium atoms |
C
|
Alpha particles have one fewer electron than helium atoms |
D
|
Alpha particles are smaller in size than helium atoms |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 52 |
Go |
Q:
|
Which of the following particles has no atomic weight? |
|
A
|
alpha |
B
|
beta |
C
|
gamma |
D
|
delta |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 53 |
Go |
Q:
|
Beta decay affects which of the following? |
|
A
|
mass number only |
B
|
atomic number only |
C
|
mass number and atomic number |
D
|
neither mass number nor atomic number |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 54 |
Go |
Q:
|
In an experiment, a radioactive metal atom experiences alpha decay and the alpha particles are collected as a gas. If the gas volume collected contains 2 x 10-6 moles, how many total atoms decayed? |
|
A
|
12 x 1018 atoms
|
B
|
6 x 1020 atoms
|
C
|
1.2 x 1019 atoms
|
D
|
1.2 x 1018 atoms
|
|
|
|
Tags:
Quantitative Skills | Nuclear Chemistry | Gases | |
|
| 55 |
Go |
Q:
|
Alpha decay of 22288Ra would produce which of the following? |
|
A
|
21886Rn |
B
|
22087Fr |
C
|
22490Th |
D
|
22286Rn |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 56 |
Go |
Q:
|
The nuclear reaction 5928Ni + e- → 5927Co + ve is an example of what type of reaction? |
|
A
|
alpha decay |
B
|
β+ decay |
C
|
electron capture |
D
|
β- decay |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 57 |
Go |
Q:
|
Radioactive decay involving gamma emission requires which of the following? |
|
A
|
Emission of a neutron. |
B
|
Emission of a proton. |
C
|
Emission of a positron. |
D
|
Gamma emission does not require any loss of particles from the nucleus. |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 58 |
Go |
Q:
|
In alpha decay, the mass number of the initial atom can be expected to: |
|
A
|
rise by 4. |
B
|
decrease by 4. |
C
|
rise by 2. |
D
|
decrease by 2. |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 59 |
Go |
Q:
|
Which of the following types of nuclear decay do NOT experience a change in mass number?
I. beta decay
II. positron emmision
III. gamma decay |
|
A
|
I and II only |
B
|
II and III only |
C
|
III only |
D
|
I, II and III |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 60 |
Go |
Q:
|
The mechanism behind electron capture may be described as: |
|
A
|
an inner shell electron combining with a proton in the nucleus. |
B
|
addition of external electron to the inner shell of an atom. |
C
|
addition of external electron to the outer shell of an atom. |
D
|
expulsion of a proton in exchange for an external electron. |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 61 |
Go |
Q:
|
Which of the following particles has the highest mass? |
|
A
|
neutrons |
B
|
gamma particles |
C
|
alpha particles |
D
|
beta particles |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 62 |
Go |
Q:
|
A scientist begins with 120kg of a radioactive substance. If the halflife of this substance is 5 minutes and the scientist waits 30 minutes, approximately how much of the substance will be remaining? |
|
A
|
2000mg |
B
|
2000g |
C
|
3000g |
D
|
3kg |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 63 |
Go |
Q:
|
A radioactive compound has a half life of 50 years. After how many years would 200g of this compound decay to to 3.125g? |
|
A
|
250 years |
B
|
300 years |
C
|
350 years |
D
|
400 years |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 64 |
Go |
Q:
|
One mole of a radioactive isotope is examined over the course of 10 halflives. How many atoms of this isotope would be expected to be left after these 10 halflives? |
|
A
|
6 x 104 |
B
|
6 x 108 |
C
|
6 x 1014 |
D
|
6 x 1020 |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 65 |
Go |
Q:
|
A particular radioactive isotope is monitored and it is noted that its mass number decreases by 64 over the course of a decay process that only involves alpha decay. How many alpha particles were released during this decay? |
|
|
|
|
Tags:
Nuclear Chemistry | |
|
| 66 |
Go |
Q:
|
Which of the following is the halflife of a first-order reaction with a rate constant of 1.4 x 10-5? |
|
A
|
0.7 x 10-2 seconds |
B
|
5 x 104 seconds |
C
|
2 x 105 seconds |
D
|
3 x 102 seconds |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 67 |
Go |
Q:
|
Gamma rays are electromagnetic radiation which involves the: |
|
A
|
release of neutrons. |
B
|
release of protons. |
C
|
release of electrons. |
D
|
release of photons. |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 68 |
Go |
Q:
|
Which of the following is true for the reaction to convert C-14 to N-14? |
|
A
|
an alpha particle is emitted |
B
|
carbon absorbs a beta particle |
C
|
beta decay takes place |
D
|
only gamma decay takes place |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 69 |
Go |
Q:
|
A radiactive source is placed at one end of a closed container. On the other end is a several-meter thick inert material. Various particles are expelled from the radioactive substance and the penetrance number, defined as the length the particle traverses across the inert material divided by the total length of the material. Which of the following particles would be expected to have the lowest penetrance number? |
|
A
|
alpha particle |
B
|
beta particle |
C
|
gamma ray |
D
|
positron emission particle |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 70 |
Go |
Q:
|
Alpha decay most commonly takes place in atoms with a large proton to neutron ratio. Which of the following atoms has the lowest proton to neutron ratio? |
|
A
|
Po-209 |
B
|
Po-210 |
C
|
Pb-210 |
D
|
Pb-209 |
|
|
|
Tags:
Nuclear Chemistry | |
|
| 71 |
Go |
Q:
|
A mummified tree is discovered and scientists attempt to determine the age of this artifact. Trees typically contain 80 mg of a particular radioactive isotope, whereas this tree contains 1 mg. The half-life of the isotope is 80,000 years. Approximately old is this artifact? |
|
A
|
1,000 years |
B
|
250,000 years |
C
|
500,000 years |
D
|
650,000 years |
|
|
|
Tags:
Nuclear Chemistry | |
|
|
Questions? We're here to help!
Ask Us
|