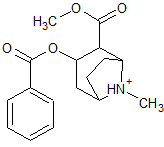
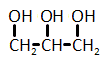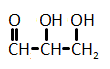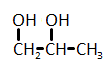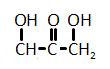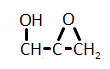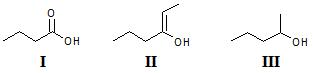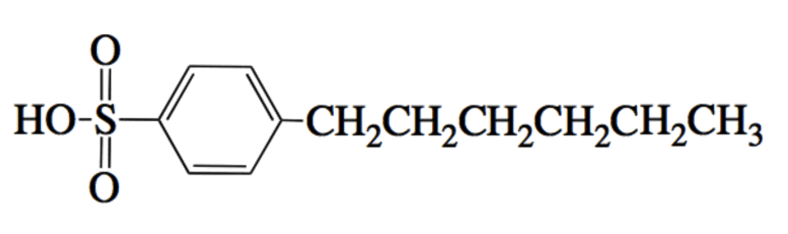|
| 1 |
Go |
Q:
|
Which of the following compounds is expected to have the lowest vapor pressure? |
|
A
|
CH3OCH3 |
B
|
CH3CH2OH |
C
|
CH3CH2CH3 |
D
|
CH3Cl |
|
|
|
Tags:
Alcohols | Gases | Molecular Bonding | |
|
| 2 |
Go |
Q:
|
Arrange the following compounds in order of increasing solubility in ethanol.
I.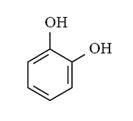
II.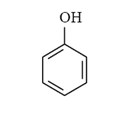
III.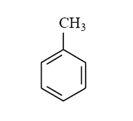
|
|
A
|
I < II < III |
B
|
II < I < III |
C
|
III < I < II |
D
|
III < II < I |
|
|
|
Tags:
Solutions | Alcohols | |
|
| 4 |
Go |
Q:
|
RNA can be seen to degrade if left at room temperature for too long, while DNA does not have this issue. The major reason for this is that |
|
A
|
The DNA backbone provides additional stability that the RNA backbone does not provide. |
B
|
The replacement of thymine with uracil decreases the stability of the RNA molecule. |
C
|
DNA is wound more tightly around itself in a different manner than RNA, providing it increased stability. |
D
|
DNA is a more stable molecule than RNA |
|
|
|
Tags:
Nucleic Acid Structure and Function | Alcohols | |
|
| 5 |
Go |
Q:
|
During atomic absorption spetroscopy, the concentration of certain atoms can be measured in very low concentrations. The absorption is tuned to the optimal absorption wavelength of individual metals. Will atomic absorption spectroscopy be useful for the measurement of ethanol in pure water? |
|
A
|
Yes because a mixture of ethanol and water does not have metal ions |
B
|
Yes because ethanol and water mixtures contain metal ions |
C
|
No because a mixture of ethanol and water does not have metal ions |
D
|
No because ethanol and water mixtures contain metal ions |
|
|
|
Tags:
Electromagnetics | Solutions | Alcohols | |
|
| 6 |
Go |
Q:
|
The catalytic hydrogenation of glycerol (shown below) is most likely to produce which of the listed compounds?
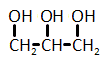 |
|
|
|
|
Tags:
Alcohols | |
|
| 8 |
Go |
Q:
|
An alcohol that is placed in concentrated acid and heat forms equilibrium with what? |
|
A
|
alkyne + water |
B
|
alkene + water |
C
|
alkyne + alkane |
D
|
alkane + alkene |
|
|
|
Tags:
Alcohols | Chemical Equilibrium | |
|
| 9 |
Go |
Q:
|
The reduction of a 3° alcohol produces what type of structure? |
|
A
|
an aldehyde |
B
|
an alkyne |
C
|
an alkane |
D
|
a 2° alcohol |
|
|
|
Tags:
Alcohols | |
|
| 11 |
Go |
Q:
|
The reaction of a single alcohol with the following compound produces a(n):
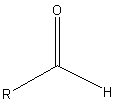 |
|
A
|
acetal |
B
|
hemiacetal |
C
|
ketal |
D
|
hemiketal |
|
|
|
Tags:
Aldehydes and Ketones | Alcohols | |
|
| 12 |
Go |
Q:
|
The molecule with the formula C7H14O2 could be which of the following types of compounds?
I. Ester
II. Carboxylic Acid
III. Alcohol (diol) |
|
A
|
I and II only |
B
|
I and III only |
C
|
II and III only |
D
|
I, II, and III |
|
|
|
Tags:
Alcohols | Carboxylic Acids | |
|
| 13 |
Go |
Q:
|
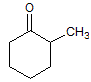
Conversion of a ketone to a secondary alcohol is which type of reaction? |
|
A
|
Oxidation |
B
|
Reduction |
C
|
Alkylation |
D
|
Dehydrogenation |
|
|
|
Tags:
Aldehydes and Ketones | Alcohols | |
|
| 14 |
Go |
Q:
|
An epoxide reacts with water under acidic conditions to form a: |
|
A
|
ether |
B
|
diol |
C
|
ester |
D
|
aromatic ring |
|
|
|
Tags:
Alcohols | |
|
| 15 |
Go |
Q:
|
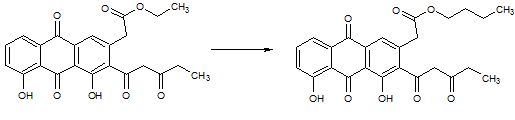
In the reaction above, which of the following compounds is used to prepare the product? |
|
A
|
MgBrCH2CH3 |
B
|
CH3CH2OCH2CH3 |
C
|
CH3CH2COOH |
D
|
CH3CH2CH2CH2OH |
|
|
|
Tags:
Alcohols | |
|
| 16 |
Go |
Q:
|
Alcohols typically have higher boiling points than ketones of similar molecular weight because alcohols |
|
A
|
are more reactive than ketones. |
B
|
are capable of hydrogen bonding. |
C
|
lack symmetry around the carbonyl carbon. |
D
|
lack carbonyl carbon atoms. |
|
|
|
Tags:
Aldehydes and Ketones | Alcohols | Intermolecular Forces | |
|
| 17 |
Go |
Q:
|
Tosylates (CH3C6H4SO2-) can be used as a protecting group for alcohols by replacing the hydroxyl with an -O-Ts group which can then be reverted to a hydroxyl group and thus preserves the alcohol. Reacting an alcohol with tosyl chloride would likely have which of the following effects on the molecule: |
|
A
|
lowering the atomic mass |
B
|
decreasing its acidity |
C
|
increasing its solubility |
D
|
all of the above are expected results |
|
|
|
Tags:
Alcohols | |
|
| 18 |
Go |
Q:
|
A student is discerning whether a reaction involving an alkane proceeds through an SN1 or an SN2 mechanism. Upon studying the products, the student concludes the reaction is proceeding through an SN1 mechanism. Which of the following pieces of evidence could the student be using to make this claim? |
|
A
|
The reaction produces a racemic mixture. |
B
|
The reaction produces an alkene. |
C
|
The reaction takes place under basic conditions. |
D
|
The primary reactant is a secondary alkyl group. |
|
|
|
Tags:
Alcohols | |
|
| 19 |
Go |
Q:
|
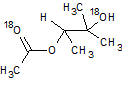
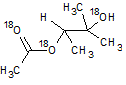
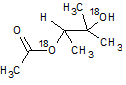
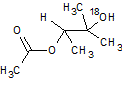
In the reaction below, if the reactant hydroxyl groups are replaced with -18OH groups, where will the 18O be found in the product?
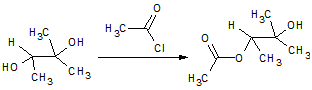 |
|
|
|
|
Tags:
Alcohols | |
|
| 20 |
Go |
Q:
|
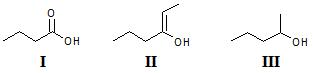
Rank the compounds above in increasing order of acidity. |
|
A
|
I < II < III |
B
|
III < II < I |
C
|
III < I < II |
D
|
I < III < II |
|
|
|
Tags:
Alcohols | Carboxylic Acids | |
|
| 21 |
Go |
Q:
|
Which of the following would be expected of the product of the reaction between acetic acid and SOCl2? |
|
A
|
The product will boil at a lower temperature than acetic acid. |
B
|
The product will form hydrogen bonds. |
C
|
The product will lose its carbonyl. |
D
|
The product and acetic acid will be inseparable using distillation. |
|
|
|
Tags:
Alcohols | Molecular Structure | |
|
| 22 |
Go |
Q:
|
What is the name of the following molecule?
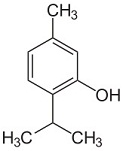 |
|
A
|
5-methyl-2-isopropylphenol |
B
|
6-isopropyl-3-methylphenol |
C
|
2-isopropyl-5-methylphenol |
D
|
3-isopropyl-6-methylphenol |
|
|
|
Tags:
Alcohols | |
|
| 23 |
Go |
Q:
|
A chemical compound is insoluble in water. Upon reaction with NaOH, however, the compound becomes soluble. What has most likely happened to the chemical compound? |
|
A
|
An -O- group or a -COO- group has been protonated |
B
|
An -OH or a -COOH group has been ionized |
C
|
A hydrogen from an alkyl group has been lost to OH- to make H2O and a water soluble carbanion |
D
|
A hydrogen from an alkyl group has been lost to OH- to make H2O and a water soluble carbocation |
|
|
|
Tags:
Acid/Base Equilibria | Alcohols | Carboxylic Acids | |
|
| 25 |
Go |
Q:
|
A compound with the molecular formula C4H10O can contain which of the following functional groups? |
|
A
|
aldehyde |
B
|
ester |
C
|
alcohol |
D
|
ketone |
|
|
|
Tags:
Alcohols | |
|
| 26 |
Go |
Q:
|
Acid- and base-catalyzed substitution reactions of alcohols differ primarily in that: |
|
A
|
acid-catalyzed reactions make better nucleophiles while base-catalyzed reactions make better leaving groups. |
B
|
acid-catalyzed reactions make better electrophiles while base-catalyzed reactions make better leaving groups |
C
|
acid-catalyzed reactions make better leaving groups while base-catalyzed reactions make better electrophiles. |
D
|
acid-catalyzed reactions make better leaving groups while base-catalyzed reactions make better nucleophiles. |
|
|
|
Tags:
Alcohols | |
|
| 27 |
Go |
Q:
|
Which of the following reactions can be expected under strongly reducing conditions? |
|
A
|
CH3CHO → CH3COOH |
B
|
CH3CH3 → CH2CH2 |
C
|
CH3COCH3 → CH3CH(OH)CH3 |
D
|
CH3CH2NH2 → CH3CN |
|
|
|
Tags:
Aldehydes and Ketones | Alcohols | |
|
| 28 |
Go |
Q:
|
The structure of 1,1-dichloroethane is shown below.
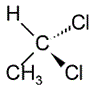
If the chemical above is treated with the Grignard reagent CH3MgBr followed by a treatment with NaOH, the major organic product would be a(n):
|
|
A
|
ketone |
B
|
primary alcohol |
C
|
aldehyde |
D
|
secondary alcohol |
|
|
|
Tags:
Alcohols | |
|
| 29 |
Go |
Q:
|
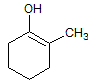
Enols are generally stronger acids than alkyl alcohols of similar structure because: |
|
A
|
enols have a lower molecular weight |
B
|
enols have more electron-donating characteristic |
C
|
enols can resonance stabilize the deprotonated form |
D
|
enols are more reactive than alkyl alcohols |
|
|
|
Tags:
Acid/Base Equilibria | Alcohols | |
|
| 30 |
Go |
Q:
|
Given the two similar molecules below, which will have the lower pka after deprotonation of the alcohol group and why?
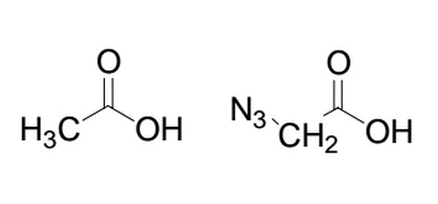 |
|
A
|
The left molecule because the carbon stabilizes the conjugate base better than the nitrogen group |
B
|
The left molecule because the nitrogen group is electron-withdrawing, which stabilizes the negatively charged conjugate base |
C
|
The right molecule because the nitrogen group will accept a proton, stabilizing the conjugate base |
D
|
The right molecule because the nitrogen group is electron-withdrawing, which stabilizes the negatively charged conjugate base |
|
|
|
Tags:
Acid/Base Equilibria | Alcohols | |
|
| 31 |
Go |
Q:
|
The molecule below is developed in the laboratory. A separate molecule is constructed where the NO2 group moves to the adjacent carbon atom. This alcohol is then deprotonated. The pka would be expected to:
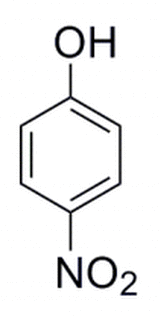 |
|
A
|
Increase because the modification allows for better resonance to stabilize the negative charge on the conjugate base |
B
|
Decrease because the modification allows for better resonance to stabilize the negative charge on the conjugate base |
C
|
Increase because the modification eliminates the resonance stabilization of the negative charge on the conjugate base |
D
|
Decrease because the modification eliminates the resonance stabilization of the negative charge on the conjugate base |
|
|
|
Tags:
Acid/Base Equilibria | Alcohols | |
|
| 32 |
Go |
Q:
|
Given the two phenols below, which phenol is expected to have the lower pKa and why? The pKa refers to the alcohol site for deprotonation.
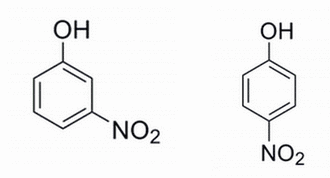 |
|
A
|
The molecule on the right will have the lower pKa and increased resonance stabilization
|
B
|
The molecule on the right will have the lower pKa and decreased resonance stabilization
|
C
|
The molecule on the left will have the lower pKa and increased resonance stabilization
|
D
|
The molecule on the left will have the lower pKa and decreased resonance stabilization
|
|
|
|
Tags:
Acid/Base Equilibria | Alcohols | |
|
| 33 |
Go |
Q:
|
An alkene with water will be in equilibrium with the alcohol that results from the hydration of the alkene by the water. With dilute acid and low temperatures, this equilibrium would be driven in which direction? |
|
A
|
To the alkene and water |
B
|
To the alcohol |
C
|
Neither side |
D
|
The direction of favoured equilibrium cannot be determined |
|
|
|
Tags:
Chemical Equilibrium | Alcohols | |
|
| 34 |
Go |
Q:
|
In each molecule below, a carbocation is formed when the alcohol group is removed. Which of the following best describes the stabilities of the carbocations?
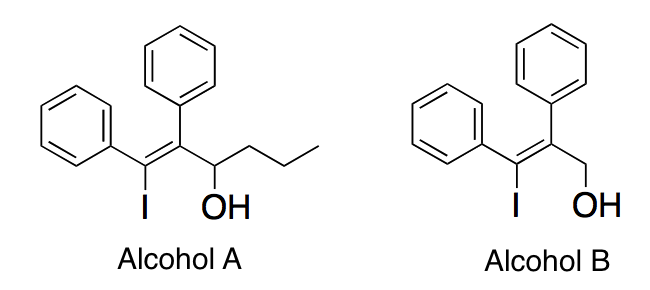 |
|
A
|
The carbocation of Alcohol A is more stable than the carbocation of Alcohol B since it is non-resonance stabilized
|
B
|
The carbocation of Alcohol A is less stable than the carbocation of Alcohol B since it is resonance de-stabilized
|
C
|
The carbocation of Alcohol B is more stable than the carbocation of Alcohol A since it is a resonance stabilized primary carbocation
|
D
|
The carbocation of Alcohol B is less stable than the carbocation of Alcohol A since it is a resonance stabilized primary carbocation
|
|
|
|
Tags:
Molecular Structure | Alcohols | |
|
| 36 |
Go |
Q:
|
Two different alcohols (R1 and R2) undergo a transesterification reaction using the same reactant, as shown below. Which of the following best explains the most likely product yields?
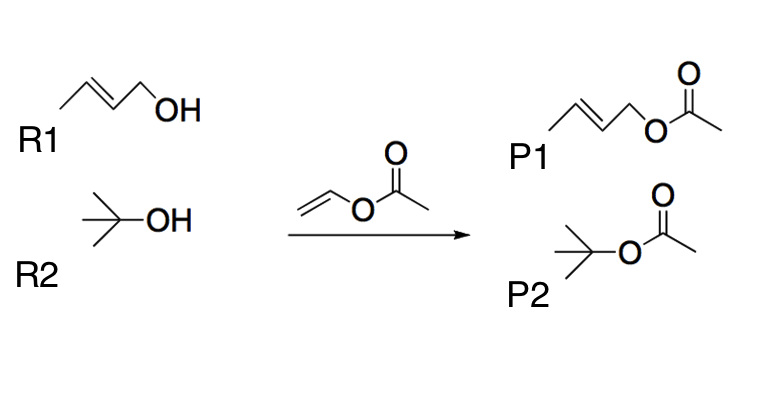 |
|
A
|
P1 will have the least yield because R1 is a primary alcohol and will not undergo a transesterification reaction.
|
B
|
P1 will have the most yield because R1 is a primary alcohol and is the least sterically hindered.
|
C
|
P2 will have the most yield because R2 is a tertiary alcohol and is the most sterically hindered.
|
D
|
P2 will have the most yield because R2 is the most basic alcohol.
|
|
|
|
Tags:
Aldehydes and Ketones | Alcohols | Molecular Structure | |
|
| 37 |
Go |
Q:
|
Given the molecule below, which of the following is TRUE regarding its ability (or inability) to undergo an acylation reaction?
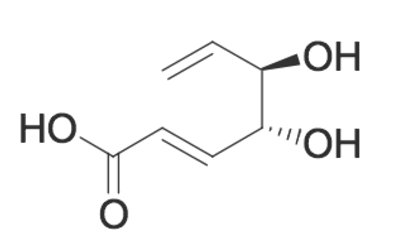 |
|
A
|
The molecule will undergo an intramolecular acylation reaction between the wedged alcohol and the carboxylic acid
|
B
|
The molecule will undergo an intramolecular acylation reaction between the dashed alcohol and the carboxylic acid
|
C
|
The molecule will NOT undergo an intramolecular acylation reaction because the double bond does not allow for the interaction between the alcohols and the carboxylic acid
|
D
|
The molecule will NOT undergo an intramolecular acylation reaction because acylation reactions cannot occur with carboxylic acids.
|
|
|
|
Tags:
Carboxylic Acids | Organic Chemistry Reactions | Molecular Structure | Alcohols | |
|
| 38 |
Go |
Q:
|
A researcher uses the molecule below as an acid catalyst to enable a reaction between a carboxylic acid and an alcohol. The reaction is designed to occur in water. Due to the relative insolubilities of the two reactants, the acid catalyst shown below can help. Which of the following best explains this catalyst's course of action?
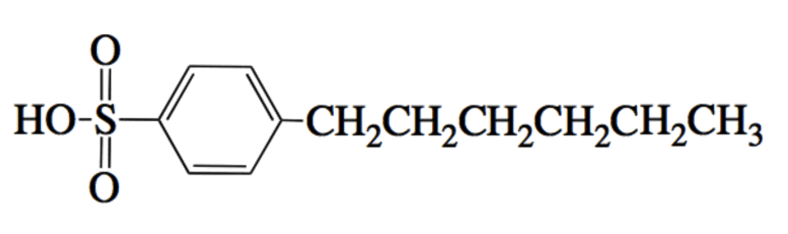 |
|
A
|
The acid catalyst forms a micelle with its long chain hydrocarbons pointed outward. This allows the acid and alcohol to react in the presence of water.
|
B
|
The acid catalyst forms a micelle with its long chain hydrocarbons pointed inward. This provides an internal environment for the acid and alcohol molecules.
|
C
|
The acid catalyst provides an attractive leaving group with its long chain hydrocarbons.
|
D
|
The acid catalyst provides many acidic protons along its long chain hydrocarbon.
|
|
|
|
Tags:
Alcohols | |
|
| 41 |
Go |
Q:
|
What difference would be expected between 1-butanol and 1,2-butanediol? |
|
A
|
1,2-butanediol would have higher volatility than 1-butanol. |
B
|
1,2-butanediol would a lower molecular weight than 1-butanol. |
C
|
1,2-butanediol would have a higher boiling point than 1-butanol. |
D
|
1,2-butanediol would have lower solubility in water than 1-butanol. |
|
|
|
Tags:
Intermolecular Forces | Alcohols | |
|
| 42 |
Go |
Q:
|
Which of the following alcohols would be expected to have the lowest pKa? |
|
A
|
1-octanol |
B
|
2-octanol |
C
|
3-octanol |
D
|
4-octanol |
|
|
|
Tags:
Alcohols | |
|
| 43 |
Go |
Q:
|
Compared with similar length non-aromatic alcohols, phenols are known to be: |
|
A
|
more basic given delocalization of charges in the conjugate base. |
B
|
more acidic given delocalization of charges in the conjugate base. |
C
|
more basic given delocalization of the hydrogen bonded to the oxygen. |
D
|
more acidic given delocalization of the hydrogen bonded to the oxygen. |
|
|
|
Tags:
Alcohols | |
|
| 44 |
Go |
Q:
|
Reacting a carboxylic acid with an alcohol in the presence of an acid catalyst would be expected to produce a(n): |
|
A
|
amide. |
B
|
ester. |
C
|
secondary alcohol. |
D
|
ketone. |
|
|
|
Tags:
Carboxylic Acids | Alcohols | |
|
| 45 |
Go |
Q:
|
Which of the following would be expected to have the highest Ka? |
|
A
|
propanol |
B
|
1-butanol |
C
|
2-butanol |
D
|
phenol |
|
|
|
Tags:
Alcohols | |
|
| 46 |
Go |
Q:
|
Which of the following molecules would be expected to have the lowest boiling point? |
|
A
|
propane |
B
|
propanol |
C
|
butane |
D
|
2-butanol |
|
|
|
Tags:
Intermolecular Forces | Alcohols | |
|
| 47 |
Go |
Q:
|
When comparing phenols and non-aromatic alcohols, which of the following is more acidic? |
|
A
|
phenols given delocalization of charges in the conjugate base
|
B
|
non-aromatic alcohols given delocalization of charges in the conjugate base |
C
|
phenols given delocalization of charges in the conjugate acid |
D
|
non-aromatic alcohols given delocalization of charges in the conjugate acid |
|
|
|
Tags:
Alcohols | |
|
|
Questions? We're here to help!
Ask Us
|