|
| 1 |
Go |
Q:
|
Elemental analysis of a compound shows that it is composed of 85% Carbon and 15% Hydrogen by mass. What is the most likely identity of the compound? |
|
A
|
C3H6
|
B
|
C3H8 |
C
|
C4H8 |
D
|
Impossible to determine with the information given. |
|
|
|
Tags:
Periodic Table | Quantitative Skills | |
|
| 2 |
Go |
Q:
|
Which of the following statements is true about the difference between sodium and magnesium? |
|
A
|
The atomic radius of Sodium is smaller than that of Magnesium |
B
|
The second ionization energy of Sodium is greater than that of Magnesium |
C
|
Sodium as a higher nuclear charge than Magnesium |
D
|
Magnesium has more core electrons than Sodium |
|
|
|
Tags:
Periodic Table | |
|
| 3 |
Go |
Q:
|
Research takes place on a different planet which contains entirely different elements from the planet Earth. The researcher constructs a periodic table based off of the extraterrestrial elements based on valence electrons (similar to the Earth periodic table). The researcher examines several atoms, and hopes to qualitatively describe their sizes. Of the following, for which position on the extraterrestrial periodic table would the largest element be found, assuming the size is only measured as atomic radius. |
|
A
|
Period II, Group III |
B
|
Period II, Group IX |
C
|
Period IV, Group III |
D
|
Period IV, Group IX |
|
|
|
Tags:
Periodic Table | |
|
| 4 |
Go |
Q:
|
An alkene is brominated at two points, resulting in a final molar mass of 202. What is the molecular formula of the starting compound assuming the only functional group is an alkene? |
|
A
|
C2H6 |
B
|
C2H8 |
C
|
C3H8 |
D
|
C3H6 |
|
|
|
Tags:
Periodic Table | |
|
| 5 |
Go |
Q:
|
Which of the following statements is true regarding 24195Am? |
|
A
|
The isotope has 95 neutrons |
B
|
The isotope has 146 neutrons |
C
|
The isotope has 241 neutrons |
D
|
The isotope has 241 electrons |
|
|
|
Tags:
Periodic Table | |
|
| 6 |
Go |
Q:
|
What is the molecular weight the compound formed by the condensation of 3 NH2CH2COOH, assuming each subunit is joined by a single bond? |
|
A
|
171 amu |
B
|
189 amu |
C
|
207 amu |
D
|
225 amu |
|
|
|
Tags:
Periodic Table | |
|
| 8 |
Go |
Q:
|
Organic molecules with a high degree of conjugation typically produce colors in solution. Which of the following also generally produce a colored solution? |
|
A
|
Strong acids and strong bases |
B
|
Elements with p orbitals and no d orbitals |
C
|
Dissolved gases |
D
|
Transition elements |
|
|
|
Tags:
Periodic Table | Solutions | |
|
| 9 |
Go |
Q:
|
Elemental analysis of a compound shows its carbon content by mass is ~50%, its oxygen content by mass is ~33%, and its hydrogen content by mass is ~15% by mass. Which of the following empirical formulas is the closest to the empirical formula of the compound? |
|
A
|
C2H6O |
B
|
C2H4O |
C
|
C3H8O |
D
|
C3H6O |
|
|
|
Tags:
Periodic Table | Stoichiometry | |
|
| 10 |
Go |
Q:
|
Technetium, element number 43, is not found naturally occurring. This suggests that technetium |
|
A
|
oxidizes very quickly |
B
|
reacts irreversibly with its surrounding environments |
C
|
cannot be produced |
D
|
has no stable isotopes |
|
|
|
Tags:
Periodic Table | |
|
| 12 |
Go |
Q:
|
A compound was analyzed and found to have 12.0 g carbon, 2.0 g hydrogen and 16.0 g oxygen. The molar mass of this compound is then determined to be 300.0 g. What is the molecular formula of the compound? |
|
A
|
C6H3O6
|
B
|
CH2O |
C
|
C12H6O12 |
D
|
C10H20O10 |
|
|
|
Tags:
Periodic Table | Stoichiometry | |
|
| 13 |
Go |
Q:
|
The mineral KAlSi3O8 has a net oxidation state of 0. What is the oxidation state of Al in the mineral? |
|
A
|
0 |
B
|
+2 |
C
|
+3 |
D
|
+2 or +3, depending on the oxidation state of the oxygen atoms |
|
|
|
Tags:
Periodic Table | |
|
| 14 |
Go |
Q:
|
Which is a feature of noble gases? |
|
A
|
They are polyatomic |
B
|
They are unstable |
C
|
They have a low boiling temperature |
D
|
Their outer shell of electrons is relatively empty |
|
|
|
Tags:
Gases | Periodic Table | |
|
| 15 |
Go |
Q:
|
Which element can form diatomic, non polar molecules? |
|
A
|
Nitrogen |
B
|
Manganese |
C
|
Sodium |
D
|
Helium |
|
|
|
Tags:
Gases | Periodic Table | |
|
| 16 |
Go |
Q:
|
Which is a feature of alkali metals? |
|
A
|
They have a single electron in their valence shell |
B
|
They have the highest ionization enthalpies in each period |
C
|
This group lies in the d-block of the periodic table |
D
|
They have a high melting point |
|
|
|
Tags:
Atomic & Electronic Structure | Periodic Table | |
|
| 17 |
Go |
Q:
|
A gaseous hydrocarbon at STP occupies 44.8 L of space. If it is measured that there is 116 g of the gas, what is the hydrocarbon? |
|
A
|
C3H8
|
B
|
C4H10 |
C
|
C5H12 |
D
|
C5H10 |
|
|
|
Tags:
Gases | Periodic Table | |
|
| 18 |
Go |
Q:
|
What is the empirical formula of a compound that is 1.59% H, 76.19% O, and 22.22% N by mass? |
|
A
|
H3NO3 |
B
|
HNO2 |
C
|
HNO3 |
D
|
HN2O4 |
|
|
|
Tags:
Periodic Table | |
|
| 19 |
Go |
Q:
|
Rank the following elements in increasing order of their reactivity with fluorine:
I. Carbon
II. Lithium
III. Cesium
IV. Oxygen |
|
A
|
IV < I < II < III |
B
|
I < III < IV < II |
C
|
III < II < I < IV |
D
|
IV < I < III < II |
|
|
|
Tags:
Periodic Table | |
|
| 21 |
Go |
Q:
|
Which of the following is not a general property of nonmetals? |
|
A
|
Low ionization energy |
B
|
Diversity of physical states |
C
|
Poor conductivity |
D
|
High electron affinity |
|
|
|
Tags:
Periodic Table | |
|
| 22 |
Go |
Q:
|
Ionization energy increases with decreasing atomic radius because |
|
A
|
the nuclear radius increases, pulling the electrons more strongly because of the proximity and increasing the energy required to ionize the atom. |
B
|
decreasing atomic radius requires higher energy orbitals which command a higher energy input during ionization. |
C
|
the number of electrons in atoms increases, meaning stronger repulsive forces against energy inputted to ionize the atoms. |
D
|
the electrons are closer to the strong attractive forces of the nucleus and require a higher energy input to be removed. |
|
|
|
Tags:
Periodic Table | |
|
| 23 |
Go |
Q:
|
Rank the following elements in increasing order of their reactivity with fluorine.
I. Potassium
II. Calcium
III. Iron |
|
A
|
I < II < III |
B
|
III < II < I |
C
|
I < III < II |
D
|
III < I < II |
|
|
|
Tags:
Periodic Table | |
|
| 24 |
Go |
Q:
|
What is the empirical formula of a compound that is composed of 60.8% Na, 28.6% B, and 10.5% H by mass? |
|
A
|
NaB2H6 |
B
|
NaB2H4 |
C
|
NaBH4 |
D
|
Na2BH8 |
|
|
|
Tags:
Periodic Table | Quantitative Skills | |
|
| 25 |
Go |
Q:
|
A carbonyl carbon acts an electrophile because |
|
A
|
the difference in electronegativity between oxygen and carbon is enough to polarize the bond |
B
|
it has sp2 hybridization |
C
|
oxygen can donate electron density to stabilize the positively-charged electrophile |
D
|
all carbon atoms act as electrophiles |
|
|
|
Tags:
Periodic Table | Molecular Bonding | |
|
| 26 |
Go |
Q:
|
Which of the following is the correct oxidation state for Cr in Cr2O72- |
|
|
|
|
Tags:
Periodic Table | |
|
| 27 |
Go |
Q:
|
Which of the following best explains the unreactivity of noble gasses? |
|
A
|
They possess an even number of electrons which is a stable configuration. |
B
|
Their proton:neutron ratio is optimal for stability. |
C
|
They have complete valence shells and do not easily accept or donate electrons to form bonds. |
D
|
Noble gasses lack d orbitals, thereby substantially increasing their stability. |
|
|
|
Tags:
Gases | Periodic Table | |
|
| 28 |
Go |
Q:
|
Which of the following pairs of elements would have the highest reactivity? |
|
A
|
H and O |
B
|
C and O |
C
|
H and Cl |
D
|
Na and F |
|
|
|
Tags:
Periodic Table | |
|
| 30 |
Go |
Q:
|
The standard atomic weight for chlorine is a weighted average of the mass of all stable isotopes and is approximately 35.5 g/mol. Using this information alone, which of the following must be true? |
|
A
|
There is exactly one stable isotope of chlorine. |
B
|
There is more than one stable isotope of chlorine. |
C
|
There are exactly two stable isotopes of chlorine. |
D
|
All isotopes of chlorine are present in roughly equal abundance. |
|
|
|
Tags:
Periodic Table | |
|
| 31 |
Go |
Q:
|
The valence band of an atom is the highest range of electron energies in which electrons are normally present. The conduction band is the range of energies where electrons are free enough to move delocalized from atom to atom. However, not all elements have electrons that lie within their conduction bands. Which of the following elements would be expected to have its valence band and its conduction bands overlapping? |
|
|
|
|
Tags:
Atomic & Electronic Structure | Periodic Table | |
|
| 34 |
Go |
Q:
|
What is the byproduct of free radical chlorination of an alkane? |
|
|
|
|
Tags:
Periodic Table | |
|
| 35 |
Go |
Q:
|
Osarizawaite is a mineral with the chemical formula PbCuAl2(SO4)2(OH)6. What is the percent mass of Aluminum in osarizawaite? |
|
|
|
|
Tags:
Periodic Table | Quantitative Skills | |
|
| 37 |
Go |
Q:
|
Which of the following has the most positive oxidation state of carbon? |
|
A
|
C2H6 |
B
|
CHCl3 |
C
|
CH4 |
D
|
C2H2 |
|
|
|
Tags:
Periodic Table | |
|
| 38 |
Go |
Q:
|
Which of the following pairs of elements would have the most reactive reaction? |
|
A
|
C and H |
B
|
Cs and Cl |
C
|
Ba and S |
D
|
B and H |
|
|
|
Tags:
Periodic Table | |
|
| 39 |
Go |
Q:
|
Dry ice (solid CO2) has a density of 1.5 g/cm3. By what factor does the volume change during the sublimation of dry ice to gaseous CO2 at STP? |
|
|
|
|
Tags:
Gases | Periodic Table | |
|
| 40 |
Go |
Q:
|
What is the percent mass of Bismuth in C7H5BiO4? |
|
|
|
|
Tags:
Periodic Table | |
|
| 42 |
Go |
Q:
|
A cobalt (II) chloride can form multiple hydrates. Which of the following is the correct molecular formula for a cobalt (II) chloride hydrate with a molecular mass of 238 g/mol? |
|
A
|
CoCl2 • H2O
|
B
|
CoCl2 • 2H2O |
C
|
CoCl2 • 4H2O |
D
|
CoCl2 • 6H2O |
|
|
|
Tags:
Periodic Table | Quantitative Skills | |
|
| 43 |
Go |
Q:
|
Elements in a periodic table group share the same number of: |
|
A
|
electrons |
B
|
valence electrons |
C
|
protons |
D
|
orbitals |
|
|
|
Tags:
Periodic Table | |
|
| 44 |
Go |
Q:
|
Which of the following elements would react most strongly with oxygen? |
|
|
|
|
Tags:
Periodic Table | |
|
| 45 |
Go |
Q:
|
Which of the following elements would have the highest first ionization energy? |
|
|
|
|
Tags:
Periodic Table | |
|
| 46 |
Go |
Q:
|
An iron nail was immersed in a solution of copper sulfate and when it was removed from the solution, it was coated with a layer of copper metal. After a different iron nail was immersed in a solution of zinc nitrate and removed, this nail rusted just as rapidly as a new iron nail. Rank these three metals in order of activity from most active to least active. |
|
A
|
Zn > Cu > Fe |
B
|
Fe > Cu > Zn |
C
|
Zn > Fe > Cu |
D
|
Cu > Fe > Zn |
|
|
|
Tags:
Periodic Table | |
|
| 48 |
Go |
Q:
|
A particular nitrogen oxide is comprised of 64% N by mass. Which of the following is a possible empirical formula for the oxide? |
|
|
|
|
Tags:
Periodic Table | Quantitative Skills | |
|
| 49 |
Go |
Q:
|
Phosphine (PH3) and ammonia (NH3) both have three hydrogen atoms because: |
|
A
|
phosphorous and nitrogen are similar in their physical properties. |
B
|
phosphorous and nitrogen are in the same group on the periodic table. |
C
|
phosphorous and nitrogen both have stable isotopes. |
D
|
phosphorous and nitrogen are both very electronegative and form bonds with hydrogen. |
|
|
|
Tags:
Periodic Table | |
|
| 50 |
Go |
Q:
|
Poor metals are the metallic elements found in the p-groups of the periodic table but are in fact metals and not metalloids. Which of the following would be the easiest way to distinguish a poor metal sample from a metalloid sample? |
|
A
|
color of the raw sample |
B
|
conductivity of the samples |
C
|
number of valence electrons |
D
|
highest electron orbital |
|
|
|
Tags:
Periodic Table | |
|
| 51 |
Go |
Q:
|
EDTA is a chelating agent that can help bind and remove multivalent toxic metals from the blood, including lead. If an individual is administered EDTA for lead poisoning, which of the following would be an important side effect to consider? |
|
A
|
Lowering of blood sodium levels. |
B
|
Lowering of blood potassium levels. |
C
|
Lowering of blood calcium levels. |
D
|
Lowering of blood chloride levels. |
|
|
|
Tags:
Periodic Table | |
|
| 53 |
Go |
Q:
|
Metallic character results from an element's ability to lose electrons. On the periodic table it is expected that metallic character increases: |
|
A
|
From left to right, because the decrease in atomic radius would result in more stable positive ion. |
B
|
From right to left, because the decrease in ionization energy would make it easier to lose electrons. |
C
|
From left to right, because the decrease in electronegativity would make it easier to lose electrons. |
D
|
From right to left, because the decrease in electron affinity would result in more stable positive ion. |
|
|
|
Tags:
Periodic Table | |
|
| 54 |
Go |
Q:
|
Which of the following increases down a group on the periodic table? |
|
A
|
number of valence electrons |
B
|
ratio of protons to electrons |
C
|
ionization energy |
D
|
metallic characteristic |
|
|
|
Tags:
Periodic Table | |
|
| 56 |
Go |
Q:
|
Isoelectronicity is an important consideration for atoms and ionic structure. Which of the following is isoelectronic to unionized argon? |
|
|
|
|
Tags:
Periodic Table | |
|
| 57 |
Go |
Q:
|
Which of the following will react most vigorously with water at room temperature? |
|
|
|
|
Tags:
Periodic Table | |
|
| 58 |
Go |
Q:
|
Electronegativity is best described as: |
|
A
|
the energy required to remove an electron. |
B
|
the degree of negative charge on an electron. |
C
|
the ability of an atom to attract electrons towards itself via a covalent bond. |
D
|
the energy change that occurs when an electron is accepted in order to form an anion. |
|
|
|
Tags:
Periodic Table | |
|
| 59 |
Go |
Q:
|
Which of the following describe the amount of attraction of one atom for electrons when bonding with another atom?
|
|
A
|
Ionic bond |
B
|
Nonpolar covalent bond |
C
|
Electronegativity |
D
|
Ionization energy |
|
|
|
Tags:
Periodic Table | |
|
| 60 |
Go |
Q:
|
Which of the following best describes the type of bond formed when sodium and chlorine react? |
|
A
|
Dispersion bond
|
B
|
Ionic bond |
C
|
Polar covalent bond
|
D
|
Nonpolar covalent bond
|
|
|
|
Tags:
Periodic Table | Molecular Bonding | |
|
| 61 |
Go |
Q:
|
Which of the following does NOT have a lower electronegativity than sulfur?
|
|
A
|
Phosphorus |
B
|
Argon |
C
|
Sodium |
D
|
Oxygen |
|
|
|
Tags:
Periodic Table | |
|
| 63 |
Go |
Q:
|
Given the molecule below, what are the oxidation numbers on the left and right carbons (of the double bond) respectively?
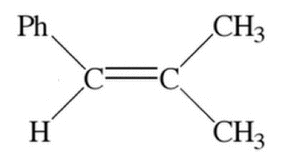 |
|
A
|
0, 0 |
B
|
-1, 0
|
C
|
0, -1
|
D
|
-1, -1
|
|
|
|
Tags:
Periodic Table | |
|
| 64 |
Go |
Q:
|
Of the following atoms listed, which has a 2s orbital closest to its nucleus?
|
|
A
|
Phosphorus |
B
|
Sulfur |
C
|
Chlorine |
D
|
Argon |
|
|
|
Tags:
Periodic Table | |
|
| 65 |
Go |
Q:
|
In describing the periodic trends of atomic radii, electron affinity, and first ionization energy, which of the following is correct going from left to right? |
|
A
|
The radius increases, the electron affinity decreases, and the first ionization energy increases
|
B
|
The radius increases, the electron affinity increases, and the first ionization energy decreases
|
C
|
The radius decreases, the electron affinity increases, and the first ionization energy increases
|
D
|
The radius decreases, the electron affinity decreases, and the first ionization energy increases
|
|
|
|
Tags:
Periodic Table | Atomic & Electronic Structure | |
|
| 66 |
Go |
Q:
|
Which of the following choices is the best description as to why alkali metals are generally more reactive than alkaline earth metals? |
|
A
|
Alkali metals have greater electron affinities than alkaline earth metals |
B
|
Alkali metals have lower densities than alkaline earth metals |
C
|
Alkali metals have lower ionization energies than alkaline earth metals |
D
|
Alkali metals have higher melting points than alkaline earth metals |
|
|
|
Tags:
Periodic Table | |
|
| 68 |
Go |
Q:
|
Which of the following orders carbon, silicon, and fluorine based on first ionization energies? |
|
A
|
Silicon > carbon > fluorine
|
B
|
Fluorine > carbon > silicon
|
C
|
Silicon > fluorine > carbon
|
D
|
Carbon > fluorine > silicon
|
|
|
|
Tags:
Atomic & Electronic Structure | Periodic Table | |
|
| 69 |
Go |
Q:
|
Which of the following represents the correct ground state electronic configuration for Fe3+? |
|
A
|
1s22s22p63s23p63d6
|
B
|
1s22s22p63s23p63d34s2
|
C
|
1s22s22p63s23p63d44s1
|
D
|
1s22s22p63s23p63d5
|
|
|
|
Tags:
Atomic & Electronic Structure | Periodic Table | |
|
| 70 |
Go |
Q:
|
Which of the following is true regarding the mass number for an atom? |
|
A
|
It is equivalent to the number of protons. |
B
|
It is equivalent to the sum of the protons and electrons. |
C
|
It differs for different isotopes of the same atom. |
D
|
It decreases going leftward along a group on the periodic table. |
|
|
|
Tags:
Periodic Table | |
|
| 71 |
Go |
Q:
|
Which of the following would be expected to have the least first ionization energy? |
|
|
|
|
Tags:
Periodic Table | |
|
| 72 |
Go |
Q:
|
Which of the following is the correct mass percentage of propane with respect to hydrogen. |
|
|
|
|
Tags:
Periodic Table | |
|
| 73 |
Go |
Q:
|
Which of the following correctly lists the electron configuration for chromium? |
|
A
|
[Ar]4s23d4 |
B
|
[Ar]4s13d5 |
C
|
[Kr]4s23d4 |
D
|
[Kr]4s13d5 |
|
|
|
Tags:
Periodic Table | |
|
| 74 |
Go |
Q:
|
When going from left to right on the periodic table, it is generally expected that: |
|
A
|
ionization energy decreases, electron affinity decreases. |
B
|
ionization energy decreases, electron affinity increases. |
C
|
ionization energy increases, electron affinity increases. |
D
|
ionization energy increases, electron affinity decreases. |
|
|
|
Tags:
Periodic Table | |
|
| 75 |
Go |
Q:
|
The atomic number of an element refers to: |
|
A
|
the number of electrons of an atom. |
B
|
the number of protons of an atom. |
C
|
the number of neutrons of an atom. |
D
|
the sum of protons and neutrons of an atom. |
|
|
|
Tags:
Periodic Table | |
|
| 76 |
Go |
Q:
|
Which of the following elements has the highest electronegativity? |
|
|
|
|
Tags:
Periodic Table | |
|
| 77 |
Go |
Q:
|
How many valence electrons do elements in group 13 typically have? |
|
|
|
|
Tags:
Periodic Table | |
|
| 78 |
Go |
Q:
|
Which of the following atoms is expected to have the highest effective nuclear charge on the 1s electron? |
|
|
|
|
Tags:
Periodic Table | |
|
| 79 |
Go |
Q:
|
Which of the following relationships holds for electron affinity? Assume absolute values for electron affinity. |
|
A
|
Affinity decreases from left to right across a period and increases downward across groups. |
B
|
Affinity decreases from left to right across a period and decreases downward across groups. |
C
|
Affinity increases from left to right across a period and increases downward across groups. |
D
|
Affinity increases from left to right across a period and decreases downward across groups. |
|
|
|
Tags:
Periodic Table | |
|
| 80 |
Go |
Q:
|
The electron configuration [Ne]3s23p6 is the structure of: |
|
A
|
krypton. |
B
|
sulfur atom. |
C
|
argon cation. |
D
|
chloride anion. |
|
|
|
Tags:
Periodic Table | |
|
| 81 |
Go |
Q:
|
Which of the following is the approximate percent composition by mass of oxygen in chromium (IV) oxide? |
|
|
|
|
Tags:
Periodic Table | |
|
| 82 |
Go |
Q:
|
Which of the following is false regarding ionization energy? |
|
A
|
it represents energy to remove a valence electron from an atom |
B
|
it measures the energy that it would take to produce a cation from a gaseous atom |
C
|
it decreases when going from left to right within a period |
D
|
it decreases with going up to down across a group |
|
|
|
Tags:
Periodic Table | |
|
| 83 |
Go |
Q:
|
Which of the following elements lives within the d-block of the periodic table? |
|
A
|
lithium |
B
|
chromium |
C
|
selenium |
D
|
aluminum |
|
|
|
Tags:
Periodic Table | |
|
| 84 |
Go |
Q:
|
Which of the following is false regarding the halogen group of elements? |
|
A
|
they readily form salt compounds |
B
|
atomic radius increases going down the group |
C
|
they have very high electronegativity |
D
|
they only have an oxidation number of -1 |
|
|
|
Tags:
Periodic Table | |
|
| 85 |
Go |
Q:
|
In which orbitals does copper contain valence electrons? |
|
A
|
4s |
B
|
3d |
C
|
3d and 4s |
D
|
3d and 4p |
|
|
|
Tags:
Periodic Table | |
|
| 86 |
Go |
Q:
|
Which of the following correctly orders from least to greatest the electronegativity of the following elements?
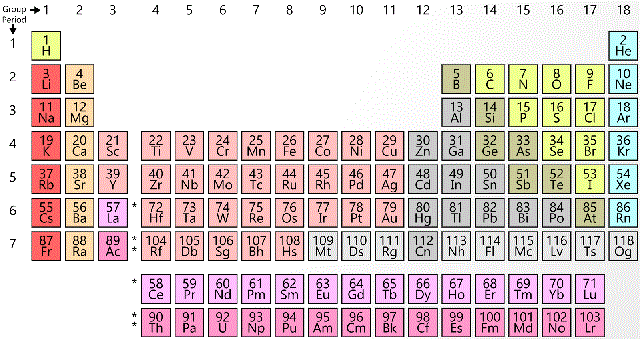 |
|
A
|
Cs < Li < I < F |
B
|
F < Li < I < Cs |
C
|
Cs < I < Li < F |
D
|
Li < I < F < Cs |
|
|
|
Tags:
Periodic Table | |
|
|
Questions? We're here to help!
Ask Us
|