|
| 1 |
Go |
Q:
|
Which of the following ways would not increase the solubility of a gas in a liquid? |
|
A
|
Increase pressure of the system |
B
|
Decrease temperature of the system |
C
|
Increase temperature of the system |
D
|
Decrease volume of the system |
|
|
|
Tags:
Fluids | Gases | |
|
| 2 |
Go |
Q:
|
In the accompanying phase diagram, a liquid can be present at combinations of temperature and pressure corresponding to the points
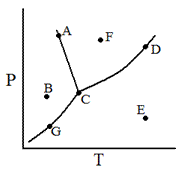 |
|
A
|
A, B, and G |
B
|
A, C, G, and D |
C
|
G, C, D, and E |
D
|
A, C, D, and F |
|
|
|
Tags:
Fluids | |
|
| 3 |
Go |
Q:
|
Arrange the following substances in terms of increasing molar entropy at standard room temperature and pressure: Ca(s), Cl2(g), Cl2(l), CaCl2(s) |
|
A
|
Cl2(g), Cl2(l), Ca(s), CaCl2(s) |
B
|
Cl2(g), Cl2(l), CaCl2(s), Ca(s) |
C
|
CaCl2(s), Ca(s), Cl2(l), Cl2(g) |
D
|
Ca(s), CaCl2(s), Cl2(l), Cl2(g) |
|
|
|
Tags:
Thermochemistry | Fluids | Gases | |
|
| 4 |
Go |
Q:
|
Suppose that you have an opened cylinder filled to the top with two equal quantities of fluids. Fluid A has a density of a and Fluid B has a density of b. If the height of the cylinder is x, what is the pressure of the fluid system at the bottom of the cylinder (assume that atmospheric pressure is Patm and that gravity is g)?
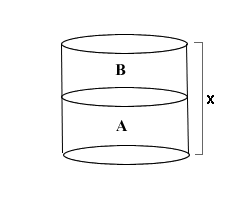 |
|
A
|
(bgx)/2 + (agx)/2 |
B
|
(bgx)/2 + agx |
C
|
Patm + (bgx)/2 + agx |
D
|
Patm + (bgx)/2 + (agx)/2 |
|
|
|
Tags:
Forces | Fluids | |
|
| 5 |
Go |
Q:
|
An unknown liquid has been isolated in a laboratory. Scientists added 100 J of heat energy with a constant source of heat. The temperature of the liquid did not increase. Which of the following could explain the reason the temperature did not increase? |
|
A
|
The liquid is at its boiling point |
B
|
The liquid is at its freezing point |
C
|
The liquid has a very low heat capacity |
D
|
The molecular bonds of the liquid do not respond to heat energy |
|
|
|
Tags:
Fluids | |
|
| 6 |
Go |
Q:
|
Which of the following molecules has the lowest vapor pressure? |
|
A
|
2-propanone |
B
|
2-butanone |
C
|
hexane |
D
|
3-octanone |
|
|
|
Tags:
Fluids | Intermolecular Forces | |
|
| 7 |
Go |
Q:
|
Which of the following compounds has the lowest vapor pressure? |
|
A
|
CH3COH |
B
|
CH3CH2COH |
C
|
CH3CH2OCH2CH3 |
D
|
HOOCCOOH |
|
|
|
Tags:
Fluids | |
|
| 8 |
Go |
Q:
|
A phase diagram depicts the conditions under which a substance is in its solid, liquid, or gaseous state. What are the axes of the phase diagram? |
|
A
|
temperature (y-axis) vs. pressure (x-axis) |
B
|
pressure (y-axis) vs. temperature (x-axis) |
C
|
temperature (y-axis) vs. vapor pressure (x-axis) |
D
|
vapor pressure (y-axis) vs. temperature (x-axis) |
|
|
|
Tags:
Fluids | Gases | |
|
| 9 |
Go |
Q:
|
A fluid moves through a tube of diameter 10 with a speed v. The fluid continues flow, and reaches a different section of the tube which has diameter 20. What is the velocity through this section of tubing? |
|
|
|
|
Tags:
Kinematics | Fluids | Quantitative Skills | |
|
| 10 |
Go |
Q:
|
Given three samples of identical mass, which is expected to have the largest volume? |
|
A
|
Solid |
B
|
Liquid |
C
|
Gas |
D
|
They should all have the same volume since they have the same mass. |
|
|
|
Tags:
Fluids | Gases | |
|
| 11 |
Go |
Q:
|
When light travels from air to water, which of the following occurs? |
|
A
|
Frequency changes |
B
|
Wavelength changes |
C
|
Energy changes |
D
|
Energy decreases but only to a minimal extent |
|
|
|
Tags:
Fluids | Gases | Electromagnetics | |
|
| 12 |
Go |
Q:
|
Which of the following will decrease the vapor pressure of a liquid? |
|
A
|
Increasing the kinetic energy of the molecules in the liquid |
B
|
Increase intramolecular hydrogen bonding |
C
|
Decrease intermolecular hydrogen bonding |
D
|
Decreasing temperature of the liquid |
|
|
|
Tags:
Fluids | |
|
| 13 |
Go |
Q:
|
A negative slope in the solid-liquid phase line in a phase diagram for a substance is indicative of |
|
A
|
The solid phase is more dense than the liquid phase |
B
|
The solid phase is less dense than the liquid phase |
C
|
The gas phase is more dense than the liquid phase |
D
|
The gas phase is more dense than the solid phase |
|
|
|
Tags:
Fluids | |
|
| 14 |
Go |
Q:
|
 In the graph between Gibbs free energy (G) and temperature (T), what is indicated at the intersection point I assuming a pressure of 1 atm?
In the graph between Gibbs free energy (G) and temperature (T), what is indicated at the intersection point I assuming a pressure of 1 atm?
|
|
A
|
The point at which an increase in temperature decreases the energetic stability of water. |
B
|
The melting point of ice. |
C
|
The point at which ice is the most energetically stable phase. |
D
|
The point at which steam is equally more entropically favorable than ice and water. |
|
|
|
Tags:
Fluids | |
|
| 15 |
Go |
Q:
|
Under standard conditions, water will pass from solid to gas through a liquid intermediate. Under which conditions will water pass directly from solid to gas without a liquid intermediate? |
|
A
|
Increased humidity |
B
|
Increased salinity |
C
|
Decreased pressure |
D
|
Decreased gravity |
|
|
|
Tags:
Fluids | Gases | |
|
| 16 |
Go |
Q:
|
Liquid is dropped from a height of 100 m. This particular liquid has a specific heat capacity of 500 J/kg*degrees Celsius.
What is the rise in liquid temperature if all potential energy was converted into heat? Assume g=10 m/s^2.
|
|
A
|
1 degrees Celsius |
B
|
2 degrees Celsius |
C
|
1.5 degrees Celsius |
D
|
5 degrees Celsius |
|
|
|
Tags:
Energy & Work | Fluids | |
|
| 17 |
Go |
Q:
|
A gas bubble is located at the bottom of a water-filled container. As the bubble is rising toward the surface, which of the following occurs? Assume the gas does not dissolve into the water. |
|
A
|
The bubble becomes smaller. |
B
|
The water level rises. |
C
|
The bubble becomes larger. |
D
|
The water level lowers. |
|
|
|
Tags:
Fluids | Gases | |
|
| 18 |
Go |
Q:
|
An unknown substance in a solid state is pressurized while keeping its temperature constant. As the pressure increases, the substance eventually converts into a liquid state. This observation suggests |
|
A
|
the solid of the substance sinks in its liquid. |
B
|
the solid of the substance is neutrally buoyant in its liquid. |
C
|
the solid of the substance floats on its liquid. |
D
|
impossible to tell from the given information. |
|
|
|
Tags:
Fluids | Gases | |
|
| 19 |
Go |
Q:
|
1 mole of a sample liquid has 10 kJ of energy added from an initial temperature of 75 degrees C. The temperature vs. energy plot is shown below. What is its heat of vaporization?
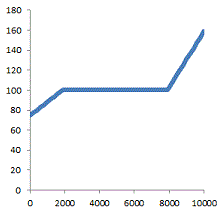 |
|
A
|
33 J/mol |
B
|
80 J/mol |
C
|
320 J/mol |
D
|
6000 J/mol |
|
|
|
Tags:
Fluids | |
|
| 20 |
Go |
Q:
|
Which of the following statements holds true for any given substance at its triple point? |
|
A
|
At the triple point, if pressure is increased, the substance will enter the liquid phase |
B
|
At the triple point, if pressure is increased, the substance will enter the solid phase |
C
|
At the triple point, if pressure is increased, the substance will enter the gas phase |
D
|
None of the above |
|
|
|
Tags:
Fluids | Gases | |
|
| 21 |
Go |
Q:
|
The vapor pressure of an unknown pure liquid is 28 mmHg. If 4 moles of nonvolatile solute are dissolved into the liquid to form 7 moles of solution, what is the vapor pressure of the solution? |
|
A
|
12 mmHg |
B
|
16 mmHg |
C
|
49 mmHg |
D
|
There is not enough information to determine the answer. |
|
|
|
Tags:
Fluids | Solutions | Quantitative Skills | |
|
| 22 |
Go |
Q:
|
A phase diagram of a newly discovered substance is constructed. For this diagram, it is found that the line that separates solid and liquid forms is perfectly vertical (i.e. has no slope). Which of the following can be deduced regarding the substance? |
|
A
|
The solid form will float on top of the liquid form |
B
|
The liquid form will float on top of the solid form |
C
|
At increased pressure, the substance will tend to the solid form |
D
|
Densities of liquid and solid form are the same |
|
|
|
Tags:
Fluids | |
|
| 23 |
Go |
Q:
|
An air bubble is released in water at a depth of 100 m. Which of the following statements is false regarding the bubble as it rises to the surface? |
|
A
|
The density of the bubble will decrease as it rises |
B
|
The bubble will maintain its volume as it rises |
C
|
The bubble will accelerate upwards as it rises |
D
|
The pressure on the bubble decreases as it rises |
|
|
|
Tags:
Fluids | Gases | |
|
| 24 |
Go |
Q:
|
Which does NOT hold true for the following phase diagram?
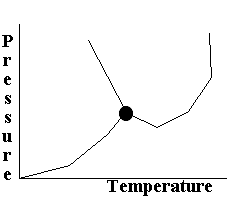
|
|
A
|
At the triple point, all three states of matter coexist |
B
|
At temperatures greater than the triple point temperature, there can exist both solid and liquid forms |
C
|
There exists a temperature/pressure combination at which only gas form can be found |
D
|
All of the above hold true |
|
|
|
Tags:
Fluids | Gases | |
|
| 25 |
Go |
Q:
|
During a phase transition from a solid to a liquid, the liquid sinks beneath the solid with the solid floating atop. On the phase diagram of such a substance, what is the slope of the line representing the liquid-solid interface? |
|
A
|
positive. |
B
|
negative. |
C
|
vertical. |
D
|
horizontal. |
|
|
|
Tags:
Fluids | |
|
| 26 |
Go |
Q:
|
The plot of air pressure versus elevation shows a nonlinear decrease while the plot of water pressure versus depth is linear. This difference is primarily due to |
|
A
|
the incompressibility of water but the compressibility of air. |
B
|
the fact that water is denser than air. |
C
|
the fact that air is more fluid than water. |
D
|
the differences in elevations measured in air height versus water depth. |
|
|
|
Tags:
Fluids | Gases | |
|
| 27 |
Go |
Q:
|
How much energy needs to be added to 2 L of water to heat it from 20 °C to 100 °C? (Note: the specific heat capacity of water is 4.184 J/g*K) |
|
A
|
669 J |
B
|
6.4 x 105 J |
C
|
6.69 x 105 J |
D
|
2.95 x 106 J |
|
|
|
Tags:
Energy & Work | Fluids | Quantitative Skills | |
|
| 28 |
Go |
Q:
|
Capillary action relies on which of the following properties to move fluids through narrow spaces? |
|
A
|
Miscibility disparity between different liquids |
B
|
Temperature disparity at different heights through the space |
C
|
Low density of liquids compared to solids |
D
|
Surface tension |
|
|
|
Tags:
Fluids | |
|
| 29 |
Go |
Q:
|
Phase transitions typically involve encountering a barrier with two distinct phases during the transition where a material must convert from one phase directly into another. This can be avoided through which of the following transitions? |
|
A
|
Gas to liquid |
B
|
Solid to liquid |
C
|
Solid to gas |
D
|
There is no way to avoid a phase transition barrier. |
|
|
|
Tags:
Fluids | Gases | |
|
| 30 |
Go |
Q:
|
What is the energy required to raise the temperature of 100 g of water from 50 °C to its boiling point and to completely vaporize it? (Assume the heat capacity of water is 4.2 J/g·K and the ΔHvaporization of water is 2260 kJ/kg) |
|
A
|
21 kJ |
B
|
247 kJ |
C
|
2281 kJ |
D
|
21226 kJ |
|
|
|
Tags:
Fluids | Gases | Quantitative Skills | |
|
| 31 |
Go |
Q:
|
Water flows from a pipe with a diameter of 40 cm into a pipe with a diameter of 4 cm. How many times faster is the water moving through the narrower pipe than through the wider pipe? |
|
A
|
√10 times faster |
B
|
10 times faster |
C
|
4 times faster |
D
|
100 times faster |
|
|
|
Tags:
Fluids | Quantitative Skills | |
|
| 32 |
Go |
Q:
|
A 1000L oil tank sits above ground and has tubing coming out of the bottom. The pressure in the tubing when no oil flows is 0.05 atm. A second tank is installed directly adjacent to the first tank and at the same height. Oil is filled to the same level as the first tank, and the tubing is joined such that both tanks flow into the tubing. What will the pressure in the tubing be? |
|
A
|
0.025 atm |
B
|
0.05 atm |
C
|
0.10 atm |
D
|
0.052 atm |
|
|
|
Tags:
Fluids | |
|
| 33 |
Go |
Q:
|
A particular liquid is brought up to boiling point. Some solute (for instance sodium chloride) is added to the solution. Which of the following would hold true? |
|
A
|
The solution will stop boiling |
B
|
The solution will boil more vigorously |
C
|
Vapor pressure of the solution will decrease |
D
|
Temperature will fall |
|
|
|
Tags:
Fluids | Solutions | |
|
| 34 |
Go |
Q:
|
A sample of water is heated by 50 degrees using 10,000 J. What is the mass of the water sample? Assume the specific heat of water is 4 J/(g*K). |
|
A
|
10 g |
B
|
40 g |
C
|
50 g |
D
|
100 g |
|
|
|
Tags:
Fluids | Quantitative Skills | |
|
| 35 |
Go |
Q:
|
The terminal velocity for an object falling through a fluid will decrease in all of the following circumstances EXCEPT: |
|
A
|
an increase in the fluid density |
B
|
an increase in the object's mass |
C
|
a decrease in the object's density |
D
|
an increase in the viscosity of the fluid |
|
|
|
Tags:
Kinematics | Fluids | |
|
| 36 |
Go |
Q:
|
A liquid with a density of 2.5 g/mL is poured over a ball to a height of 50 cm above the ball. A second liquid with a density of 1.5 g/mL, immiscible with the first liquid, is poured over the liquid to an additional height of 50 cm. What is the total pressure on the ball at the bottom? |
|
A
|
12500 N/m2 |
B
|
32500 N/m2 |
C
|
20000 N/m2 |
D
|
27500 N/m2 |
|
|
|
Tags:
Fluids | Quantitative Skills | |
|
| 37 |
Go |
Q:
|
An organism is discovered which lives in very cold environments close to 0 °C. Compared to organisms that live at room temperature, this cold-tolerant organism can be expected to: |
|
A
|
require more calories for survival |
B
|
require thermostable enzymes for maintenance of metabolic pathways |
C
|
have a high metabolic rate |
D
|
have a high solute content in its cytosol |
|
|
|
Tags:
Fluids | |
|
| 38 |
Go |
Q:
|
If the radius of a pipe doubles, how will the linear velocity of the fluid flowing through the pipe be affected if the volume flow rate remains the same? |
|
A
|
velocity will double |
B
|
velocity will quadruple |
C
|
velocity will be halved |
D
|
velocity will be quartered |
|
|
|
Tags:
Fluids | |
|
| 39 |
Go |
Q:
|
A krypton fluoride laser can emit light in brief pulses of only 4 nanoseconds. Given the velocity of light in a vacuum is 3 x 108 m/s, how long is each pulse of light if emitted underwater? Note the index of refraction of water is 1.33. |
|
A
|
90 cm |
B
|
1.2 m |
C
|
1.6 m |
D
|
2.1 m |
|
|
|
Tags:
Kinematics | Fluids | Quantitative Skills | |
|
| 40 |
Go |
Q:
|
Simple mercury barometers were historically particularly useful because the high density of mercury allows a barometer to be only approximately 1 m tall to measure normal fluctuations in atmospheric pressure, which determined the height of the mercury. If a barometer reads a height of 1000 mmHg, what height would be read on a barometer using water instead? The density of mercury is 13.5 g/mL and that of water is 1 g/mL. |
|
A
|
74 mm |
B
|
74 cm |
C
|
135 cm |
D
|
13.5 m |
|
|
|
Tags:
Fluids | Quantitative Skills | |
|
| 41 |
Go |
Q:
|
Water droplets on a waxed car tend to be spheres as small as possible so as to: |
|
A
|
Minimize surface tension |
B
|
Maximize the area to volume ratio |
C
|
Minimize cohesive forces |
D
|
Maximize adhesive forces |
|
|
|
Tags:
Fluids | |
|
| 42 |
Go |
Q:
|
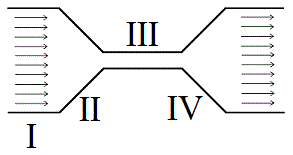
An ideal fluid flows through the system above with the same volume flow rate at all given points. At which point would the system exhibit the lowest pressure? |
|
|
|
|
Tags:
Fluids | |
|
| 43 |
Go |
Q:
|
Which of the following increases the solubility of a gas in a liquid? |
|
A
|
Increasing temperature |
B
|
Decreasing pressure |
C
|
Decreasing temperature |
D
|
Increasing volume of the container |
|
|
|
Tags:
Fluids | Gases | |
|
| 44 |
Go |
Q:
|
A container of water with a small, floating object is placed inside of an elevator. Given that only the container is in the elevator (no people), what happens to the object (compared to the water level) if the elevator accelerates upward? |
|
A
|
The acceleration effectively increases g, which results in more of the object being visible above the water line
|
B
|
The acceleration effectively increases g, which results in less of the object being visible above the water line
|
C
|
The acceleration effectively decreases g, which results in less of the object being visible above the water line
|
D
|
There is no change in the water level
|
|
|
|
Tags:
Fluids | Forces | |
|
| 45 |
Go |
Q:
|
For a science project, a student makes a hole in the side of a container holding water, which then flows out in a parabolic trajectory. The student then drops the container from the top of a building, putting it in free fall. During this fall, what will happen to the flow of water out of the hole in the side of the container? |
|
A
|
The water follows the same parabolic trajectory
|
B
|
The water flows out in a straight line
|
C
|
The water stops flowing out of the hole
|
D
|
The water flows out in a curved, upward trajectory
|
|
|
|
Tags:
Fluids | Forces | |
|
| 46 |
Go |
Q:
|
Given two hoses, with Hose A having a diameter 50% larger than Hose B. The two hoses are connected (one after the other) from a source of water, and there is no leaking. Through which section of the total hose does water flow the fastest? |
|
A
|
The Hose A section
|
B
|
The Hose B section
|
C
|
The flow is the same in either section
|
D
|
The faster flow rate depends on the order of the Hose A and Hose B sections
|
|
|
|
Tags:
Fluids | |
|
| 47 |
Go |
Q:
|
A high school science experiment is testing volume displacement and buoyant force by taping a solid weight on top of a solid styrofoam box. The box is then placed in a pool and floats. But, due to the weight on top of the box, the water line is flush with the top surface of the box ("Surface A"), so it looks like just the weight is floating (with the styrofoam box submerged under it). If the teacher flips the box over such that "Surface A" is facing downward (and "Surface B" is now towards the top), where will the new water line be?
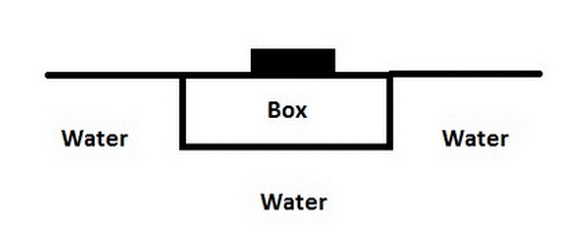
|
|
A
|
The water line will be flush with Surface B
|
B
|
Now that the weight is submerged and facing downward, the entire box will sink
|
C
|
Due to the buoyant force, the box will rotate itself back to the original arrangement
|
D
|
Now that the weight is submerged and facing downward, the water line will be between Surface A and Surface B
|
|
|
|
Tags:
Fluids | Forces | |
|
| 48 |
Go |
Q:
|
Which of the following is NOT a characteristic of an ideal fluid? |
|
A
|
Uniform density |
B
|
Incompressible |
C
|
Confined to turbulent flow |
D
|
Does not experience viscosity |
|
|
|
Tags:
Quantitative Skills | Fluids | |
|
| 49 |
Go |
Q:
|
Two containers of equal volume have the same pressure but different temperatures. Which of the following must be true of the containers? |
|
A
|
The container with the higher temperature has fewer moles of gas. |
B
|
The container with the higher temperature has more moles of gas. |
C
|
The two containers have the same number of moles of gas. |
D
|
The answer cannot be determined from the given information.
|
|
|
|
Tags:
Fluids | |
|
| 50 |
Go |
Q:
|
At a hydroelectric plant, a falling fluid turns a turbine. The moving turbine is used to run electric generators. Given this scenario, which of the following is true? |
|
A
|
The magnitude of the electrical energy produced is equal to the magnitude of the gravitational potential energy released by the falling fluid.
|
B
|
The magnitude of the electrical energy produced is less than the magnitude of the gravitational potential energy released by the falling fluid.
|
C
|
The magnitude of the electrical energy produced is greater than the magnitude of the gravitational potential energy released by the falling fluid.
|
D
|
The answer cannot be determined from the given information.
|
|
|
|
Tags:
Energy & Work | Fluids | |
|
| 51 |
Go |
Q:
|
Which of the following will increase the vapor pressure of a liquid? |
|
A
|
Decreasing the temperature of the liquid
|
B
|
Increasing the temperature of the liquid
|
C
|
Decreasing the surface area of the liquid
|
D
|
Increasing the surface area of the liquid
|
|
|
|
Tags:
Gases | Fluids | Molecular Bonding | |
|
| 52 |
Go |
Q:
|
An object is placed on a scale and its weight is recorded. How would the recorded weight of the object change as the atmospheric pressure was increased? |
|
A
|
The recorded weight would increase because the air density increases as pressure increases. |
B
|
The recorded weight would increase because air density decreases as pressure increases. |
C
|
The recorded weight would decrease because the air density increases as pressure increases. |
D
|
The recorded weight would decrease because the air density decreases as pressure increases. |
|
|
|
Tags:
Gases | Fluids | Forces | |
|
| 53 |
Go |
Q:
|
Vasoconstriction of a blood vessel would have which of the following affects at the site of constriction, assuming the volume of blood passing through does not change? |
|
A
|
The velocity of the blood flow would increase. |
B
|
The cross-sectional area of the vessel would increase. |
C
|
Constriction involves muscle relaxation. |
D
|
The rate of gas exchange in the region would increase. |
|
|
|
Tags:
Fluids | Circulatory System | |
|
| 54 |
Go |
Q:
|
The volume of blood that fits into a 10 cm section of an artery with a diameter of 0.5 cm would fit into a venule with a diameter of 0.1 cm of what length? |
|
A
|
25 cm |
B
|
50 cm |
C
|
500 cm |
D
|
250 cm |
|
|
|
Tags:
Fluids | Circulatory System | |
|
| 55 |
Go |
Q:
|
A lead sphere with a volume of 1 mL and a silicon sphere with a volume of 1 mL are dropped into water. What is the buoyant force on each sphere? Note: the density of lead is 11 g/mL and the density of silicon is 2.3 g/mL. |
|
A
|
The buoyant force is 0.11 N on the lead sphere and 0.023 N on the silicon sphere. |
B
|
The buoyant force is 0.00091 N on the lead sphere and 0.0043 N on the silicon sphere. |
C
|
The buoyant force on both spheres 11 N. |
D
|
The buoyant force on both spheres is 0.01 N. |
|
|
|
Tags:
Fluids | Forces | |
|
| 56 |
Go |
Q:
|
Which of the following would NOT increase the pressure at the bottom of a fluid-filled cylinder? |
|
A
|
increasing the force of gravity |
B
|
replacing the fluid with a higher-density fluid to the same level |
C
|
increase the height of the fluid |
D
|
increasing the diameter of the cylinder and filling it to the same height it was previously |
|
|
|
Tags:
Fluids | |
|
| 57 |
Go |
Q:
|
For a wave of a particular frequency f, which of the following correctly lists the wavelengths of the wave as it passes through solid, liquid, and gas? |
|
A
|
wavelength(solid) > wavelength(liquid) > wavelength(gas) |
B
|
wavelength(gas) > wavelength(liquid) > wavelength(solid) |
C
|
wavelength(solid) > wavelength(gas) > wavelength(liquid) |
D
|
wavelength(gas) > wavelength(solid) > wavelength(liquid) |
|
|
|
Tags:
Fluids | |
|
| 58 |
Go |
Q:
|
A cube with dimensions 2cm x 2cm x 2cm has a density of 1.2 g/cm3 and is placed in water. What volume of the cube would expected to be below below the surface? |
|
A
|
1 cm3 |
B
|
4.5 cm3 |
C
|
7.6 cm3 |
D
|
8 cm3 |
|
|
|
Tags:
Fluids | |
|
| 59 |
Go |
Q:
|
Which of the following is true for a fluid that forms a convex surface when in a test tube? |
|
A
|
cohesive forces are stronger than adhesive forces |
B
|
adhesive forces are stronger than cohesive forces |
C
|
cohesive and adhesive forces are identical |
D
|
there is absence of both cohesive and adhesive forces in this system |
|
|
|
Tags:
Fluids | |
|
| 60 |
Go |
Q:
|
A large cylindrical tube of height 80m is filled with water. A hole is punctured in the middle of the tube. Which of the following lists the approximate velocity of the fluid exiting at the puncture site? |
|
A
|
10 m/s |
B
|
20 m/s |
C
|
30 m/s |
D
|
40 m/s |
|
|
|
Tags:
Fluids | |
|
| 61 |
Go |
Q:
|
An object has the density of 0.75 g/cm3 and is placed in a pool of water. Which of the following lists the fraction of the object that rests above the water at equilibrium? |
|
|
|
|
Tags:
Fluids | |
|
| 62 |
Go |
Q:
|
Two volatile liquids, A and B, are combined as a solution in a closed beaker. There are 3 moles of liquid A and 9 moles of liquid B. The vapor pressure of liquid A is 10 mmHg and the vapor pressure of liquid B is 20 mmHg. Which of the following depicts the vapor pressure of the solution? |
|
A
|
16.7 mmHg |
B
|
17.5 mmHg |
C
|
19.5 mmHg |
D
|
21.5 mmHg |
|
|
|
Tags:
Fluids | |
|
| 63 |
Go |
Q:
|
Sphere A of radius R and sphere B of radius 2R both sink in a bathtub. Assuming the density of both spheres is identical, which of the following is the ratio of the buoyant force on sphere A to the buoyant force on sphere B. |
|
|
|
|
Tags:
Fluids | |
|
| 64 |
Go |
Q:
|
A skyscraper receives its water supply from the ground floor (sea level) through a pipe with diameter 10 cm with water running at a velocity of 5 m/s. On a floor 85m above the ground, a high-pressure sink expels water at a rate of 25 m/s. What is the cross-sectional area of the pipe for this sink? |
|
A
|
0.0015 m2 |
B
|
0.003 m2 |
C
|
0.0045 m2 |
D
|
0.006 m2 |
|
|
|
Tags:
Fluids | |
|
| 65 |
Go |
Q:
|
The Venturi effect involves which of the following variables? |
|
A
|
volume and pressure of a gas1 |
B
|
amplitude and velocity of a fluid wave |
C
|
diameter of a fluid molecule and diffusion speed |
D
|
fluid pressure and cross-sectional area of a pipe |
|
|
|
Tags:
Fluids | |
|
| 66 |
Go |
Q:
|
Which of the following holds true regarding an object entirely submerged in a fluid? |
|
A
|
the buoyancy force is zero |
B
|
the buoyancy force is nonzero however is not directed in the upward or downward direction |
C
|
the buoyancy force is proportional to atmospheric pressure |
D
|
fluid pressure is exerted on the entire surface of the object but is greater on the bottom of the object |
|
|
|
Tags:
Fluids | |
|
| 67 |
Go |
Q:
|
Two pipes have cross-sectional area X and connect to become a single pipe, called pipe P. The output flow in pipe P is quadruple the flow of that in the two pipes and is measured as F. What is the cross-sectional area of pipe P? |
|
|
|
|
Tags:
Fluids | |
|
| 68 |
Go |
Q:
|
Mercury acts as a liquid in room temperature. It has a velocity v when traveling through tubing of radius 5 cm. What velocity of mercury would be expected when mercury travels through tubing of radius 10 cm? |
|
|
|
|
Tags:
Fluids | |
|
| 69 |
Go |
Q:
|
The venturi effect refers to a phenomenon where: |
|
A
|
turbulence of a fluid decreases at very high velocities. |
B
|
turbulence of a fluid increases at very high velocities. |
C
|
fluid pressure increases in a constricted portion of a tube. |
D
|
fluid pressure decreases in a constricted portion of a tube. |
|
|
|
Tags:
Fluids | |
|
| 70 |
Go |
Q:
|
A spherical turkey with density D is submerged in a brine of density B. We know that D
|
|
|
|
|
Tags:
Fluids | |
|
| 71 |
Go |
Q:
|
A gas is compressed so that its volume decreases by a factor of 3. As a result, the density of the gas: |
|
A
|
decreases by a factor of 3. |
B
|
decreases by a factor of 9. |
C
|
increases by a factor of 3. |
D
|
increases by a factor of 9. |
|
|
|
Tags:
Fluids | |
|
| 72 |
Go |
Q:
|
An elementary school teacher pours a thick, slimy substance down a tube suspended above her most rambunctious student's head. Instead of running straight down through the tube onto the student's head like water would, this unknown substance slowly oozes down the tube, giving the student time to escape. Which of the following is most related to the likely reason this substance behaves this way? |
|
A
|
Volume |
B
|
Density |
C
|
Intramolecular forces |
D
|
Intermolecular forces |
|
|
|
Tags:
Fluids | |
|
| 73 |
Go |
Q:
|
Which of the following concepts best explains the reason that a hot air balloon is able to ascend into the sky? (Remember that the bottom of the hot air balloon is open so heated air may be blown in.) |
|
A
|
Air Pressure |
B
|
Density of Air |
C
|
Volume of Air |
D
|
Air Resistance |
|
|
|
Tags:
Fluids | |
|
|
Questions? We're here to help!
Ask Us
|







