|
| 1 |
Go |
Q:
|
Most proteins have optimal pH ranges. Once the pH shifts outside the optimal range, enzyme activity decreases.
The reason for this decrease is: |
|
A
|
a change in the 3-dimensional structure of the enzyme |
B
|
feedback inhibition |
C
|
allosteric inhibition |
D
|
decrease in the synthesis of the enzyme |
|
|
|
Tags:
Protein Structure and Function | Enzyme Structure and Function | |
|
| 2 |
Go |
Q:
|
In superoxide dismutase 1, an enzyme implicated in amyotrophic lateral sclerosis, a copper ion is bound to the active site and is important for enzyme activity. In this enzyme, copper most likely functions as a(n) |
|
A
|
coenzyme |
B
|
cofactor |
C
|
allosteric activator |
D
|
allosteric inhibitor |
|
|
|
Tags:
Enzyme Structure and Function | Control of Enzyme Activity | |
|
| 3 |
Go |
Q:
|
If an enzyme solution is saturated with substrate, the most effective way to obtain an even faster yield of products would be to: |
|
A
|
add more of the enzyme |
B
|
heat the solution to 90 degrees C |
C
|
add more substrate |
D
|
add an allosteric inhibitor |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 4 |
Go |
Q:
|
An overall reaction is made up of two elementary reactions. After the first step, the concentration of one particular species has increased. What could be the role of the species? |
|
A
|
Product |
B
|
Catalyst |
C
|
Intermediate |
D
|
Either A or C |
|
|
|
Tags:
Enzyme Structure and Function | Stoichiometry | |
|
| 5 |
Go |
Q:
|
In the oxidation of glucose to water and carbon dioxide, enzymes are needed to catalyze the |
|
A
|
combination of glucose molecules |
B
|
release of energy by hydrogen removal |
C
|
storage of energy in glycogen molecules |
D
|
production of lactic acid |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 6 |
Go |
Q:
|
Peroxisomes are single-membrane vesicles with high concentrations of catalase and urate oxidase. In a mutant lacking catalase, what is the most likely result in the cell? |
|
A
|
the inability to break down glycogen |
B
|
the inability to break down uric acid |
C
|
the inability to breakdown fatty acids |
D
|
the inability to breakdown accumulated peroxide |
|
|
|
Tags:
Enzyme Structure and Function | Eukaryotic Cells | |
|
| 7 |
Go |
Q:
|
In animals, enzymes and hormones are similar in that both substances |
|
A
|
do not contain the element nitrogen |
B
|
function only within cells |
C
|
are products of cell synthesis |
D
|
must be ingested |
|
|
|
Tags:
Enzyme Structure and Function | Eukaryotic Cells | |
|
| 8 |
Go |
Q:
|
An enzyme in which the addition of one substrate increases the rates at which additional substrates may bind is called a(n) |
|
A
|
proteolytic enzyme |
B
|
allosteric enzyme |
C
|
Coenzyme |
D
|
Kinase |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 9 |
Go |
Q:
|
Protein phosphatases usually do which of the following modifications? |
|
A
|
Phosphorylation |
B
|
Acetylation |
C
|
Methylation |
D
|
Dephosphorylation |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 11 |
Go |
Q:
|
Chlorophyll absorbs maximally in red and blue
wavelengths. Consider a genetically modified plant which
expresses an enzyme that emits red and blue light using
energy from the conversion of ATP to ADP. If the plant
were placed in complete darkness, would it be able to grow sustainably
from the light energy emitted by the enzymes? |
|
A
|
No, because the amount of energy absorbed by the plant
must be greater than or equal to the energy involved in
the metabolism of the plant, impossible under the
described conditions since the light energy emitted must
be less than the energy involved in the metabolism of the
plant. |
B
|
No, because the amount of energy absorbed by the plant
must be less than or equal to the energy involved in the
metabolism of the plant, yet the energy emitted by the
enzymes is greater than the the energy involved in the
metabolism of the plant.
|
C
|
Yes, because the energy emitted from the enzyme is
greater than the required energy for metabolic metabolic
processes required to produce the light.
|
D
|
Yes, because only a small amount of light energy is
needed to stimulate growth; the remainder can come from
the soil, air, and water. |
|
|
|
Tags:
Enzyme Structure and Function | Energy & Work | |
|
| 12 |
Go |
Q:
|
An enzyme is capable of changing all of the following except |
|
A
|
Activation energy |
B
|
The rate of a particular reaction |
C
|
The rate of the reverse of a particular reaction |
D
|
Enthalpy of formation of a compound |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 13 |
Go |
Q:
|
Cellulases are capable of breaking β(1→4) glycosidic bonds. Which type of enzyme is capable of breaking α(1→4) glycosidic bonds? |
|
A
|
pepsin |
B
|
amylase |
C
|
acetylesterase |
D
|
lipase |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 14 |
Go |
Q:
|
Peroxisomes are single-membrane vesicles with high concentrations of catalase and urate oxidase. In a mutant lacking catalase, what is the most likely result in the cell? |
|
A
|
the inability to break down glycogen |
B
|
the inability to break down uric acid |
C
|
the inability to break down fatty acids |
D
|
the inability to breakdown accumulated peroxide |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 15 |
Go |
Q:
|
Which of the following statements about enzymes is false? |
|
A
|
Enzymes lower the activation energy of a reaction. |
B
|
Enzymes are recycled at the end of the reaction. |
C
|
Enzymes shift reaction equilibrium towards the products. |
D
|
Enzymes exhibit saturation kinetics. |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 16 |
Go |
Q:
|
Which is true of enzymes? |
|
A
|
An apoenzyme is an active enzyme-cofactor complex |
B
|
Cofactors may be inorganic or organic |
C
|
Coenzymes are a type of tightly-bound cofactor |
D
|
Prosthetic groups are a type of loosely-bound cofactor |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 17 |
Go |
Q:
|
Which of the following is false about a catalyst? |
|
A
|
A catalyst is altered during the course of a reaction and resumes its original state after the completion of a reaction. |
B
|
The maximum reaction rate increases with the addition of more catalyst. |
C
|
The rate constant, k, of a reaction rate law remains unchanged in the presence of catalyst. |
D
|
The rate of a catalyzed reaction decreases over time if substrate is not continually supplied. |
|
|
|
Tags:
Enzyme Structure and Function | Control of Enzyme Activity | |
|
| 18 |
Go |
Q:
|
A researcher develops an enzyme inhibitor by creating an analog that is very similar to the substrate of the enzyme. However, she finds that the inhibitor lacks some important similarities to the substrate and although the structures are substantially similar, the inhibitor does not bind to the enzyme. This observation suggests what about the nature of how the enzyme binds to its substrate? |
|
A
|
The substrate forms covalent bonds to its enzyme |
B
|
The inhibitor binds allosterically to the enzyme |
C
|
The enzyme binds to its substrate using the induced fit model |
D
|
The enzyme has very low affinity for its substrate |
|
|
|
Tags:
Enzyme Structure and Function | Control of Enzyme Activity | |
|
| 19 |
Go |
Q:
|
Kinases are important enzymes because they facilitate |
|
A
|
Ketylation |
B
|
Phosphorylation |
C
|
Dehydration |
D
|
Alkylation |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 20 |
Go |
Q:
|
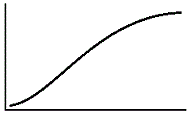
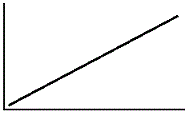
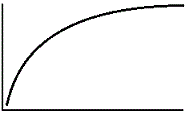
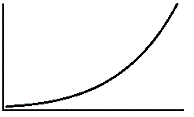
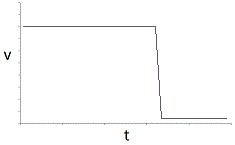
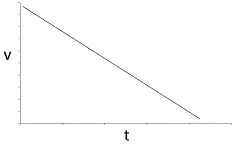
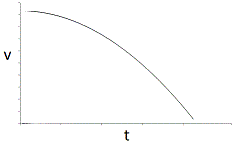
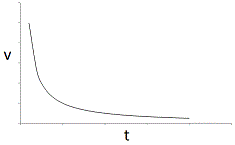
An enzyme solution is added to a solution of substrate and the concentration of product is measured from t = 0. Which of the following graphs accurately depicts the graph of the product concentration versus time? |
|
|
|
|
Tags:
Enzyme Structure and Function | Chemical Kinetics | |
|
| 22 |
Go |
Q:
|
Efavirenz is a non-competitive inhibitor of reverse transcriptase (RT) found in HIV. It is included in the treatment regimen for HIV patients. Which of the following likely does not hold true for Efavirenz? |
|
A
|
Efavirenz binds to a site outside of the the active site of RT |
B
|
Efavirenz cannot be outcompeted with RT substrate |
C
|
Increased production of RT by HIV could overcome Efavirenz therapy |
D
|
Efavirenz has a structure similar to host nucleotides |
|
|
|
Tags:
Enzyme Structure and Function | Viruses | |
|
| 23 |
Go |
Q:
|
The peptide bond in proteins is difficult to break using chemical methods under physiological conditions. Proteases are often used to increase the rate of hydrolized product formation. These enzymes serve which of the following functions?
I. Decrease the activation energy of the reaction
II. Drive the equilibirum of the reaction towards the products
III. Raise the pKa of the protein to make it more reactive |
|
A
|
I only |
B
|
I and II only |
C
|
II only |
D
|
I and III only |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 24 |
Go |
Q:
|
Horseradish peroxidases are able to catalyze a variety of reduction reactions to convert peroxides into alcohols. Other peroxidases, however, are often only able to catalyze the reduction reaction with a specific substrate. This information suggests which of the following? |
|
A
|
Horseradish peroxidases do not have negative feedback loops with their products while other peroxidases do. |
B
|
Horseradish peroxidases are less susceptible to denaturing agents than other peroxidases. |
C
|
Horseradish peroxidases have active sites that are more accessible to peroxides than other peroxidases. |
D
|
Horseradish peroxidases use induced-fit binding to their substrates while other peroxidases use lock-and-key binding. |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 25 |
Go |
Q:
|
For a drug that binds and inhibits its protein target irreversibly, which of the following would be expected? |
|
A
|
The effects of the drug last only as long as the patient is actively consuming the drug. |
B
|
The effects of the drug last for several days or longer after the patient stops taking the drug. |
C
|
The drug permanently disables the function in question and the patient no longer needs to take the drug. |
D
|
The patient becomes dependent on the drug and must not stop taking it to prevent reversal of the target activity. |
|
|
|
Tags:
Enzyme Structure and Function | Control of Enzyme Activity | |
|
| 26 |
Go |
Q:
|
Unlike enzymatic amino acid synthesis, most chemical amino acid synthesis produces racemic mixtures. The difference is primarily due to which of the following? |
|
A
|
Enzymes work at lower temperatures and are therefore able to catalyze more sensitive biochemical reactions. |
B
|
Enzymatic reactions are usually stereospecific. |
C
|
Chemical synthesis involves reagents that are racemic. |
D
|
Chemical synthesis has a higher activation energy making it more likely to produce a racemic mixture. |
|
|
|
Tags:
Protein Structure and Function | Enzyme Structure and Function | |
|
| 27 |
Go |
Q:
|
Reaction rates increase with increasing temperature, because the rate constant k is related to the absolute temperature. However, reactions catalyzed by enzymes have an upper limit on the temperatures at which they are able to catalyze reactions. This is primarily because: |
|
A
|
high temperatures denature enzymes. |
B
|
enzymes alter reaction rate kinetics. |
C
|
enzymes are unique catalysts in that the reactions they catalyze are independent of temperature. |
D
|
enzymes alter the reaction mechanism. |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 29 |
Go |
Q:
|
An enzyme catalyzes a reaction which converts A to B. This enzyme
I. Increases the forward rate of the reaction
II. Increases the reverse rate of the reaction
III. Decreases the activation energy for the reaction |
|
A
|
III only |
B
|
I and III only |
C
|
I only |
D
|
I, II, and III |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 30 |
Go |
Q:
|
A hypothetical weight-loss drug inhibits ATP Synthase. Which of the following best describes why this drug could possibly help with weight loss? |
|
A
|
Due to the drug inhibiting ATP Synthase, glucose cannot be broken down, and is therefore excreted
|
B
|
The drug blocks ATP metabolism, requiring the person to break down the energy stored in their fat reserves
|
C
|
Due to the drug inhibiting ATP Synthase, more glucose is used because less ATP can be made per molecule
|
D
|
The drug causes cells to use far less ATP than normal, so they use less glucose
|
|
|
|
Tags:
Enzyme Structure and Function | Glycolysis, Gluconeogenesis, PPP | |
|
| 32 |
Go |
Q:
|
The liver and muscle both play crucial roles in glycogen breakdown, but there are differences between the two. For example, muscle cells lack the enzyme glucose-6-phosphatase, which is required to pass glucose into the blood. Which of the following is FALSE regarding glycogen metabolism? |
|
A
|
Although the muscle stores large amounts of glycogen, the liver is the major source of circulating glucose.
|
B
|
Only the glycogen stored in the liver can be made accessible to other organs in the body
|
C
|
While the muscle stores large amounts of glycogen, it lacks an enzyme to break down glycogen and release large amounts of glucose into circulation
|
D
|
While the liver stores large amounts of glycogen, it lacks an enzyme to break down glycogen and release large amounts of glucose into circulation
|
|
|
|
Tags:
Glycolysis, Gluconeogenesis, PPP | Enzyme Structure and Function | |
|
| 33 |
Go |
Q:
|
During the first step of glucose metabolism, an enzyme drives catalysis and binds glucose in its active site. A second site exists (away from the active site) which binds ATP (the eventual end-product of glucose metabolism). Which of the following is NOT correct in describing the mechanism behind the ATP-binding site on this enzyme? |
|
A
|
The ATP-binding site is an example of feedback inhibition
|
B
|
ATP acts as a non-competitive inhibitor of the enzyme.
|
C
|
The enzyme is less active when ATP is present in high concentrations
|
D
|
The enzyme is more active when ATP is present in high concentrations
|
|
|
|
Tags:
Enzyme Structure and Function | Glycolysis, Gluconeogenesis, PPP | |
|
| 34 |
Go |
Q:
|
Protease (an HIV enzyme) contains hydrophobic residues at its active site. Which of the following would be an ideal characteristic for a drug that would act as a competitive inhibitor to protease? The drug should be: |
|
A
|
Polar |
B
|
Nonpolar |
C
|
Charged |
D
|
Neutral |
|
|
|
Tags:
Enzyme Structure and Function | Control of Enzyme Activity | |
|
| 35 |
Go |
Q:
|
Which of the following is a true statement regarding cofactors and coenzymes? |
|
A
|
Cofactors and coenzymes allow enzymes to catalyze a wider range of chemical reactions
|
B
|
Cofactors and coenzymes allow enzymes to function at lower concentrations
|
C
|
Cofactors and coenzymes maximize the ΔG
|
D
|
Cofactors and coenzymes change endergonic reactions to exergonic reactions
|
|
|
|
Tags:
Enzyme Structure and Function | Control of Enzyme Activity | |
|
| 36 |
Go |
Q:
|
Lab results from a protein analysis reveal its complete structure, and that it catalyzes the breakdown of a larger substrate. Moreover, there are two binding sites (one for the large substrate, and one for a small regulatory molecule). Which of the following best describes the mechanism by which this protein works? |
|
A
|
The protein is likely structural, and is involved in cell-to-cell adhesion
|
B
|
The protein is likely an enzyme that works through competitive inhibition
|
C
|
The protein is likely an enzyme that works through allosteric regulation
|
D
|
The protein is likely a membrane transport protein for ions
|
|
|
|
Tags:
Protein Structure and Function | Enzyme Structure and Function | |
|
| 37 |
Go |
Q:
|
Which of the following is a correct statement regarding a competitive inhibitor of an enzyme? |
|
A
|
The competitive inhibitor changes the enzyme shape by binding to a site other than the active site
|
B
|
The competitive inhibitor binds to the product of the enzymatic reaction
|
C
|
The competitive inhibitor, which resembles the shape of the substrate, binds to the active site
|
D
|
The competitive inhibitor causes the enzyme reaction to become nonspontaneous
|
|
|
|
Tags:
Enzyme Structure and Function | Control of Enzyme Activity | |
|
| 38 |
Go |
Q:
|
Which of the following is NOT true regarding enzyme-catalyzed reactions? |
|
A
|
Enzymes lower the activation energy
|
B
|
Enzymes form complexes with their substrates
|
C
|
Enzymes change the Keq for chemical reactions
|
D
|
Enzymes can change shape when the substrate binds
|
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 39 |
Go |
Q:
|
Some vitamins, like the B complex vitamins, function as precursors for enzyme cofactors. Which of the following is NOT a scenario that can involve a vitamin acting as a precursor for enzyme cofactors? |
|
A
|
A vitamin assisting enzymes in their function as catalysts in metabolism |
B
|
A vitamin enhancing absorption of certain minerals |
C
|
A vitamin covalently bonding to an enzyme and utilizing ATP |
D
|
A vitamin binding to enzyme catalysts as a coenzyme |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 40 |
Go |
Q:
|
Compared to the concentration of cations outside a nerve cell, the concentration of cations inside a nerve cell is different. Which of the following processes best explains this difference? |
|
A
|
Simple diffusion across the membrane |
B
|
Osmosis of water |
C
|
Membrane contransporters |
D
|
Sodium-potassium pumps |
|
|
|
Tags:
Nervous System | Enzyme Structure and Function | |
|
| 41 |
Go |
Q:
|
Which of the following best explains why glycolysis AND gluconeogenesis are characterized by irreversible processes in cells? |
|
A
|
The processes occur in separate cellular compartments |
B
|
All of the enzymes in both pathways are different, so the reaction pathways do not overlap |
C
|
Key reactions in each pathway are characterized by large, negative changes in free energy |
D
|
The processes are triggered by the same irreversibly-binding hormones |
|
|
|
Tags:
Glycolysis, Gluconeogenesis, PPP | Enzyme Structure and Function | |
|
| 42 |
Go |
Q:
|
Which of the following is NOT correct regarding the control and regulation of gluconeogenesis? |
|
A
|
The control of gluconeogenesis is inversely linked to the control of glycolysis
|
B
|
Glucagon stimulates adenylyl cyclase, which causes cAMP formation
|
C
|
Most of the enzymes responsible for the control and regulation of gluconeogenesis are found in the cytoplasm
|
D
|
Acetyl-CoA only activates gluconeogenesis enzymes and has no inhibitory role in gluconeogenesis
|
|
|
|
Tags:
Enzyme Structure and Function | Glycolysis, Gluconeogenesis, PPP | Control of Enzyme Activity | |
|
| 43 |
Go |
Q:
|
Which of the following is TRUE regarding a competitive inhibitor? |
|
A
|
A competitive inhibitor binds reversibly at the enzyme's active site. |
B
|
A competitive inhibitor covalently binds to the enzyme. |
C
|
A competitive inhibitor decreases the maximum velocity of the reaction. |
D
|
A competitive inhibitor binds at multiple different sites on the enzyme. |
|
|
|
Tags:
Enzyme Structure and Function | Control of Enzyme Activity | |
|
| 45 |
Go |
Q:
|
Which of the following is FALSE regarding DNA replication? |
|
A
|
DNA replication requires an RNA primer.
|
B
|
Nucleotide triphosphates are added to the 3' end.
|
C
|
Bacteria require telomerase to complete their DNA strand synthesis.
|
D
|
Helicase is required prior to DNA synthesis.
|
|
|
|
Tags:
Enzyme Structure and Function | DNA Replication and Repair | |
|
| 46 |
Go |
Q:
|
Which of the following are methods that an enzyme can use to lower activation energy?
I. Reacting with the substrate
II. Creating a stable electrostatic environment during the transition state
III. Positioning reactive groups of the enzyme and substrate to be in close proximity of each other |
|
A
|
II only
|
B
|
I and II only
|
C
|
II and III only
|
D
|
I, II, and III
|
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 47 |
Go |
Q:
|
An increase in the KM of a protein could be a result of: |
|
A
|
negative regulation, because the protein's affinity for its substrate decreases.
|
B
|
positive regulation, because the protein's affinity for its substrate decreases. |
C
|
negative regulation, because the protein's affinity for its substrate increases. |
D
|
positive regulation, because the protein's affinity for its substrate increases. |
|
|
|
Tags:
Control of Enzyme Activity | Enzyme Structure and Function | |
|
| 48 |
Go |
Q:
|
A protein exhibiting cooperativity would have the greatest sensitivity to the equilibrium of its substrate binding when: |
|
A
|
The protein is approximately 50% saturated with substrate. |
B
|
The protein is completely saturated with substrate. |
C
|
The protein is completely unsaturated with substrate. |
D
|
The protein would exhibit the same sensitivity at all times. |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 49 |
Go |
Q:
|
Which of the following is NOT a mechanism used to activate enzymes? |
|
A
|
cleavage of part of the enzyme |
B
|
phosphorylation |
C
|
dephosphorylation |
D
|
dessication |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 50 |
Go |
Q:
|
An enzyme with a low KM will: |
|
A
|
denature at a higher temperature than an enzyme with a high KM. |
B
|
reach its vmax at a lower substrate concentration than an enzyme with a high KM. |
C
|
have fewer allosteric inhibitor sites than an enzyme with a high KM. |
D
|
have a higher vmax than an enzyme with a high KM. |
|
|
|
Tags:
Enzyme Structure and Function | Control of Enzyme Activity | |
|
| 51 |
Go |
Q:
|
Which of the following is FALSE regarding enzyme inhibition? |
|
A
|
The vmax of an enzyme remains unchanged in the presence of a competitive inhibitor. |
B
|
An inhibitor that binds to an allosteric site must be non-competitive inhibitor. |
C
|
Inhibition of enzyme activity does not require a threshold concentration of inhibitor to be present. |
D
|
In uncompetitive inhibition, the inhibitor has a higher affinity for the enzyme-substrate complex than for the unbound enzyme. |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 52 |
Go |
Q:
|
Which of the following relationships for the Michaelis constant (Km) for an enzyme catalyzed reaction holds true? |
|
A
|
Increasing the substrate concentration increases Km |
B
|
Decreasing the substrate concentration increases Km |
C
|
Km represents the slope of the reaction rate vs substrate concentration graph |
D
|
Km is a particular concentration at which the reaction rate is at half of Vmax |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 53 |
Go |
Q:
|
MAP kinase kinase is an enzyme which is expected to: |
|
A
|
hydroxylate a molecule called MAP. |
B
|
add two carbonyl groups onto a molecule called MAP. |
C
|
dehosphorylate a molecule called MAP. |
D
|
phosphorylate a molecule called MAP kinase. |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 54 |
Go |
Q:
|
A reaction takes place as follows: A --> B --> C, where enzyme X acts for A --> B and enzyme Y acts for B --> C. It is discovered that C acts in addition with enzyme X to increase the reaction rate. This can be referred to as: |
|
A
|
allosteric inhibition. |
B
|
negative feedback. |
C
|
noncompetitive inhibition. |
D
|
positive feedback. |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 55 |
Go |
Q:
|
A competitive inhibitor results in: |
|
A
|
a decreased Vmax and a decreased Km. |
B
|
a decreased Km and an unchanged Vmax. |
C
|
a decreased Vmax and an increased Km. |
D
|
an unchanged Vmax and an increased Km. |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 56 |
Go |
Q:
|
A particular enzyme catalyzes a reaction with a Km of 3 x 10-3 M and Vmax of 100 mol/min. Which of the following represents the rate of the reaction when the substrate is a concentration of Km? |
|
A
|
1.5 x 10-3 mol/min |
B
|
3 x 10-3 mol/min |
C
|
50 mol/min |
D
|
100 mol/min |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 57 |
Go |
Q:
|
An uncompetitive inhibitor: |
|
A
|
only binds to the enzyme-substrate complex. |
B
|
can bind to the enzyme independent of whether the substrate is bound. |
C
|
is almost always irreversible. |
D
|
the inhibitor can only bind to an enzyme that is not bound to the substrate. |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 58 |
Go |
Q:
|
With respect to enzymatic reactions, what holds true regarding the transition state? |
|
A
|
it is a state which more closely resembles reactants than products |
B
|
it is a state which more closely resembles products than reactants |
C
|
it is always a higher energy state than both products and reactants |
D
|
it is a higher energy state than products but not necessarily reactants |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 59 |
Go |
Q:
|
A science lab studies enzyme kinetics for a particular set of enzymes and substrates. It is found that the enzyme's active site differs in conformation from the substrate however when the substrate moves in close proximity to the active site, small conformational changes take place to allow the substrate to bind. This evidence supports: |
|
A
|
the lock and key hypothesis. |
B
|
enzymatic reduction of activation energy. |
C
|
the induced-fit model. |
D
|
protease catalysis. |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 60 |
Go |
Q:
|
Which of the following is an accurate statement regarding sodium-potassium pump? |
|
A
|
the pump functions through facilitated diffusion |
B
|
ATP is not required for its function |
C
|
it moves potassium and sodium ions in equal amounts across the plasma membrane |
D
|
it moves sodium from a lower concentration to a higher concentration |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 61 |
Go |
Q:
|
Which of the following is true regarding noncompetitive inhibition? |
|
A
|
it involves an inhibitor binding to the allosteric site on an enzyme |
B
|
it decreases the Km of a reaction |
C
|
it increases the Km of a reaction |
D
|
it involves binding of the inhibitor to the active site of the enzyme |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 62 |
Go |
Q:
|
RDB phosphorylase kinase would be expected to: |
|
A
|
be a transmembrane protein. |
B
|
remove a phosphate group to an RDB protein. |
C
|
add a phosphate group to RDB phosphorylase. |
D
|
decarboxylate an organic molecule. |
|
|
|
Tags:
Enzyme Structure and Function | |
|
| 63 |
Go |
Q:
|
A mixed enzyme inhibitor is one which: |
|
A
|
results in both inhibition as well as activation of an enzyme. |
B
|
the inhibitor may bind to the enzyme irrespective of whether the substrate has bound to the enzyme. |
C
|
the inhibitor must be thoroughly intermixed throughout the enzyme/substrate solution for it to have activity. |
D
|
the enzyme only binds to the inhibitor when there is a small amount of substrate present. |
|
|
|
Tags:
Enzyme Structure and Function | |
|
|
Questions? We're here to help!
Ask Us
|